
Toshiba echo activation image showing a color-coded map of where activation starts and ends during each contraction. This example shows a posterior lateral infarction timing defect.
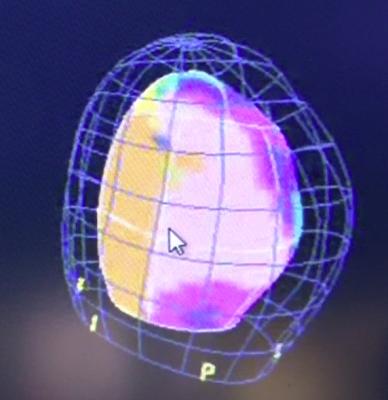
An activation imaging example showing a heart with left bundle branch block. Its believed this type of imaging will help lead to better CRT lead placement optimization.
March 15, 2013 – For better treatment planning during cardiac resynchronization therapy (CRT), Toshiba America Medical System Inc.’s Activation Imaging is the company’s latest addition to its 3-D Wall Motion Tracking software. Activation Imaging is an U.S. Food and Drug Administration (FDA)-cleared, proprietary technology available on the Aplio Artida cardiovascular ultrasound system.
When the heart is not functioning properly, different segments activate at different moments in time. Activation Imaging, in conjunction with Toshiba’s comprehensive 3-D Wall Motion Tracking software, allows clinicians to evaluate dyssynchrony at the onset of the heart’s contraction and to properly identify the left ventricle’s pumping strength and timing for more accurate lead placements in CRT treatment.
“Toshiba continues to pioneer innovative ultrasound technology to improve patient outcomes,” said Tomohiro Hasegawa, director, Ultrasound Business Unit, Toshiba. “Studies show that CRT procedures have a 33 percent failure rate.[1] Activation Imaging’s ability to measure dyssynchrony at a more appropriate moment in time, the onset of contraction, will provide greater confidence during CRT planning to improve patient outcomes and lower healthcare costs.”
Toshiba showcased Activation Imaging at this year’s American College of Cardiology (ACC) annual meeting in San Francisco, March 9 to 11.
For more information: www.medical.toshiba.com
1. Abraham WT, Fisher WG, Smith AL, et al. “MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure.” N Engl J Med 2002;346: 1845–53.



 February 05, 2026
February 05, 2026 









