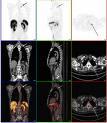
May 13, 2009 - American College of Radiology (ACR) Image Metrix has been chosen by the Academy of Molecular Imaging (AMI) to provide data and image storage infrastructure for a new study to compare the effectiveness of conventional planar 99mTc-MDP bone imaging with 18F-sodium fluoride PET/CT (18F-NaF) at detecting bony metastases in patients with breast, prostate and non–small-cell lung cancers.
The protocol was developed in conjunction with the U.S. Food and Drug Administration (FDA) and the Centers for Medicare and Medicaid Services (CMS) and calls for data collection on more than 500 patients at 13 sites nationwide.
“Just as early detection of cancer is important, early detection of any spread of a cancer is vitally important to determining the best and most timely treatment for patients. PET and PET/CT are serving an increasingly important role in this care. Each refinement in care can make a big difference in the success of treatment for additional patients. ACR Image Metrix is proud to serve such a significant role in this important trial as the selected CRO [Contract Research Organization],” said ACR Image Metrix Chief Scientific Officer Bruce J. Hillman, M.D., FACR, who worked with AMI investigators to develop the protocol for the research.
Cancer patients undergo more than 2 million planar 99mTc-MDP scans each year to determine whether cancer has spread from the organ of primary diagnosis to their bones. However, the test may miss some disease. 18F-NaF PET/CT bone scanning may have advantages over the conventional method in that it is often able to find smaller metastases and differentiate more accurately between cancerous and non-cancerous conditions.
“ACR Image Metrix is unique in the CRO landscape regarding its ability to assist in protocol development then transmit, archive, and retrieve imaging data for such important trials. We are proud of our ability to substantively contribute to advances in patient care by providing significant infrastructure for cutting edge medical research,” said Michal Morales, General Manager of ACR Image Metrix.
For more information: www.acr.org and www.ami-imaging.org


 October 30, 2025
October 30, 2025 









