September 9, 2016 — The Alzheimer’s Disease Neuroimaging Initiative (ADNI) recently launched into its next phase, ADNI3, thanks to a $40 million, five-year award from the National Institutes of Health (NIH). ADNI3 — part of the long-running NIH-supported ADNI study investigating brain and fluid biomarkers of Alzheimer’s — will couple the NIH award with anticipated private sector contributions of $20 million through the Foundation for the National Institutes of Health (FNIH).
ADNI3 will use cutting-edge technologies in brain imaging as it recruits hundreds of new volunteers. Expansion of the groundbreaking study, now in its 12th year, will further develop ways to speed clinical trials by providing researchers the biomarkers needed to detect the onset and track the progression of Alzheimer’s disease.
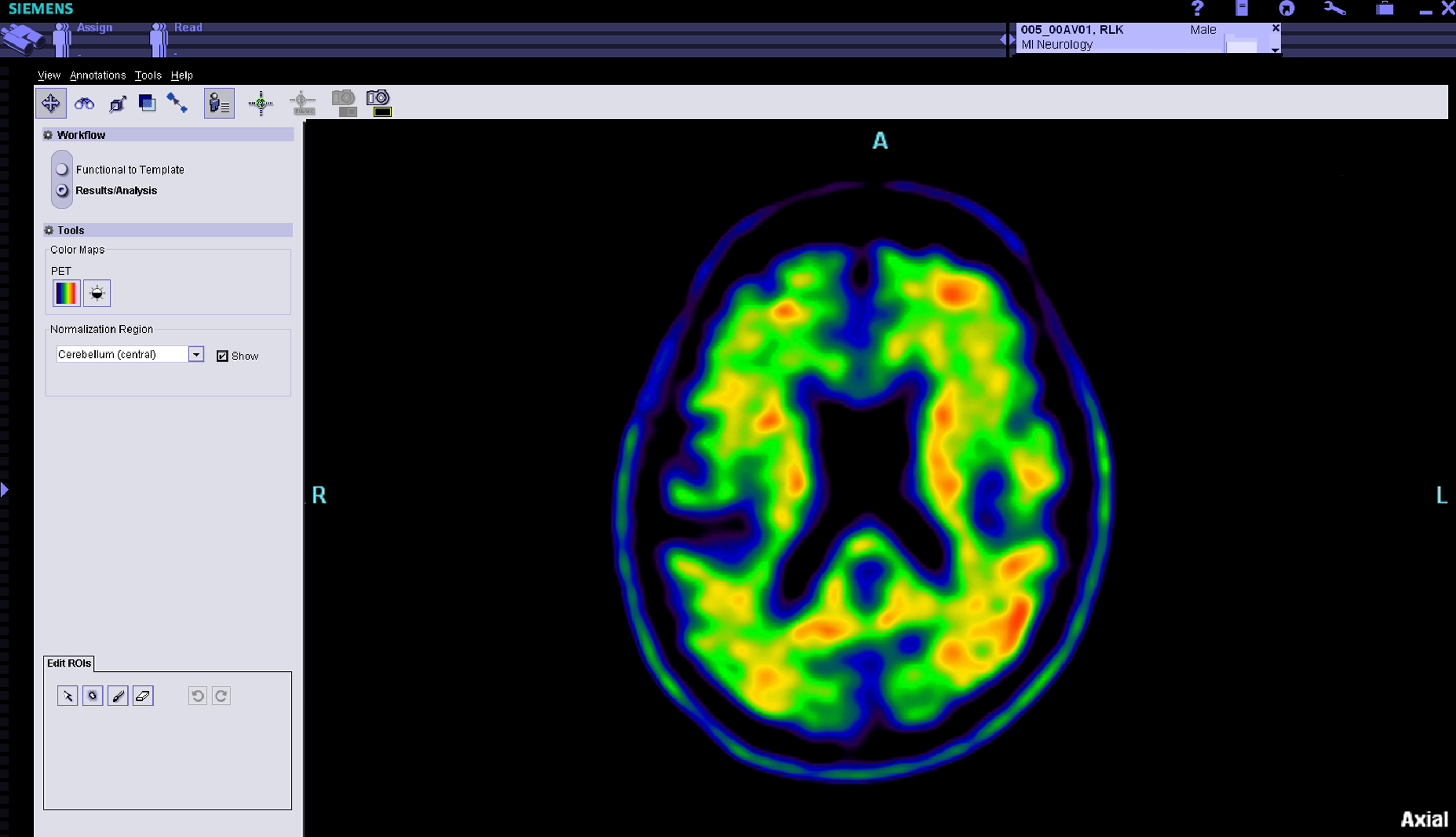
The study matches changes in clinical and cognitive testing with Alzheimer’s-related changes in biological markers detected in blood, cerebrospinal fluid and DNA samples donated by volunteers. Brain scans identify changes in brain volume, white matter integrity, functional connectivity between brain regions, glucose metabolism and the buildup of amyloid protein plaques — a hallmark of the disease. ADNI3 will add brain scans that detect tau protein tangles, another indicator of the disease. Blood, cerebrospinal fluid and DNA samples also enable scientists to better understand Alzheimer’s-related chemical changes and how genes influence the disorder.
ADNI, one of the largest public-private partnerships in Alzheimer’s disease research, is supported by the National Institute on Aging (NIA) at NIH through a grant to the nonprofit Northern California Institute for Research and Education, San Francisco. Michael Weiner, M.D., of the San Francisco Department of Veterans Affairs Medical Center and the University of California, San Francisco (UCSF), is principal investigator for the study.
By identifying and validating such biomarkers as abnormal levels of amyloid protein, ADNI has led to insights on who may be at risk for the disease and how Alzheimer’s-related brain changes correspond to clinical findings. In recent years, these game-changing discoveries have advanced clinical trial design and led to the testing of promising interventions at the earliest stages of the disease.
“ADNI3 will move the bar higher still in this collaborative effort to gain a clear understanding of the subtle Alzheimer’s-related brain changes in volunteers, long before symptoms appear, and the biological changes that mark its progression,” said NIA Director Richard J. Hodes, M.D. “These insights are vital to researchers and clinicians working worldwide in their selection of clinical trial volunteers and the testing of promising interventions.”
ADNI3 recruitment starting in the fall of 2016 will seek up to 1,200 volunteers, over the age of 55, to join about 800 current participants at 60 sites in the United States and Canada. The volunteers represent the full trajectory of the disease, including those with normal cognition, mild cognitive impairment (often a precursor to Alzheimer’s) and Alzheimer’s dementia.
“We are thrilled to embark on this next phase of discovery, enhanced by sophisticated new technologies and computational methods that we could only dream about when we launched the study in 2004,” Weiner said. “ADNI has made a profound difference in clinical trials, developing and refining the biomarker tools needed to see Alzheimer’s-related brain changes in the living brain — even in people free of symptoms. ADNI3 will play an even more influential role as these biomarkers are enlisted in the search for treatments for this devastating disorder.”
Innovative new measures and tools include:
- The use of tracers that image tau protein tangles — a significant indicator of Alzheimer’s disease — in the living brain. Researchers will gain new insights into how and where the protein builds in the brain, and how it may interact with amyloid protein plaques to drive disease progression;
- Researchers will explore how the connections between the structure and function of the brain change in Alzheimer’s disease, in part by using methods developed by the NIH-funded Human Connectome Project for magnetic resonance images (MRIs) of the brain;
- Trouble handling money is often an early warning sign of Alzheimer’s. The Financial Capacity Instrument (FCI) will assess volunteers on real-life money skills, such as mental calculation, understanding financial concepts, using a checkbook to pay bills and reading a bank statement. By validating whether declines in FCI performance can predict who is at risk for the disease among those with normal cognition or mild cognitive impairment, ADNI3 may validate a test of a real-life function that can be used in a doctor’s office and in clinical trials; and
- Researchers will recruit and track cognitive changes in volunteers online via the Brain Health Registry, a resource promoting dementia clinical trials sponsored and managed by UCSF. Participants will access the registry to periodically fill out confidential questionnaires about their health, lifestyle and medical history and take computerized tests measuring memory, attention and other measures of cognition.
ADNI3 will build upon the discoveries made during earlier phases. ADNI1 started in 2004, ADNI-GO (Grand Opportunity) began in 2009 and ADNI2 in 2010. It is also enhanced by insights gained from conducting a U.S. Department of Defense-supported study on the role of traumatic brain injury and post-traumatic stress disorder on developing Alzheimer’s in Vietnam veteran volunteers.
A key feature of ADNI has been its focus on collaboration and rapid data sharing. To speed the pace of analysis and findings, ADNI investigators make their collected data widely available; the study has been used in over 1,200 research papers. MRI and positron emission tomography (PET) scan brain images as well as clinical, genetic and fluid biomarker data are available to qualified researchers worldwide through a web-based database. To date, more than 8,500 researchers have sought access.
ADNI is a founding member of the World Wide Alzheimer's Disease Neuroimaging Initiative, an umbrella organization created and led by the Alzheimer’s Association for neuroimaging initiatives modeled after ADNI in Europe, Asia, Australia and Latin America.
Private sector funding donated to the project through the FNIH will come from pharmaceutical, imaging and clinical trial management companies and non-profit organizations. Thus far, the following organizations have supported ADNI3 by contributing funds totaling more than $14 million: AbbVie, Alzheimer’s Association, Bioclinica, Biogen, Cogstate, DiamiR Biosciences, Eisai, Genentech (a member of the Roche Group), Janssen R&D LLC, Lundbeck, Merck & Company Inc., PeopleBio Inc., Pfizer Inc., Roche, Servier and Takeda. Beyond raising funds to support the study, the FNIH also coordinates the scientific engagement of private partners through the ADNI Private Partner Scientific Board.
Study sites will begin recruiting in the fall.
For more information: www.adni.brainhealthregistry.org


 February 13, 2026
February 13, 2026 









