Last November, the U.S. Food and Drug Administration (FDA) issued a final guidance for medical imaging vendors on the information required in premarket notifications related to pediatric X-ray equipment. Optimizing imaging dose is particularly important for children, as their smaller size makes them more vulnerable and they are more likely to suffer long-term effects if overexposed. The guidance offers new recommendations for vendors on device labeling for pediatric use, and also covers the information that should be included in a device’s 510(k) submission and instructions for use.
Complications With Pediatric Dose
The FDA guidance is intended to clarify ways that vendors can help providers optimize the delivered dose in pediatric X-ray exams. There are several factors that complicate dose determinations when it comes to pediatric patients.
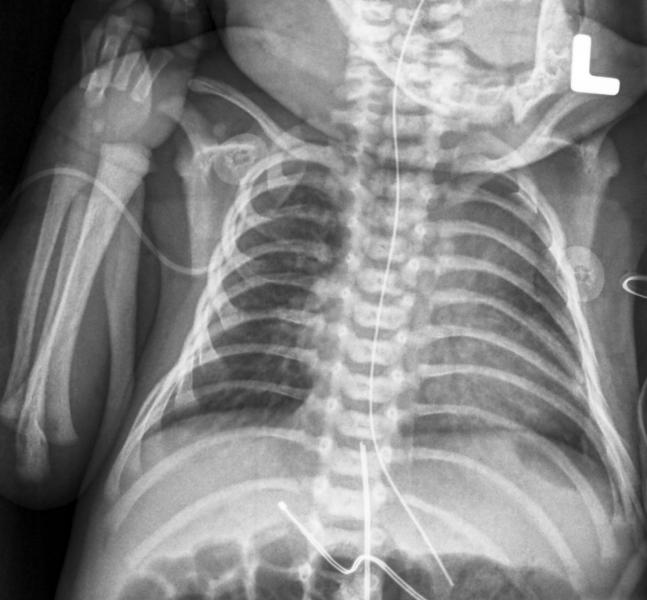
The biggest challenge is determining how to segment pediatric patients for the purpose of dose calculation. Depending on the healthcare facility, “pediatric patients” are those 18 and younger, or in some places 21 and younger. Size differences complicate things further — there is greater variation in size between the youngest and oldest pediatric patients than between the youngest and oldest “adult” patients, which complicates the management of the patient dose. The dose needed to acquire an adequate X-ray is based on the size of the part of the patient being imaged, so the dose would be different between an infant and a teenager. “And how do you even quantify the size of the pediatric patient?” said Keith Strauss, MSc, FAAPM, FACR, associate professor of radiology at the University of Cincinnati School of Medicine and head of the clinical imaging physics section of the radiology department at Cincinnati Children’s Hospital. Strauss is also vice-chair of the Image Gently Alliance.
Beyond patient factors, pediatric X-ray dosing is also a function of where the exams are performed and the imaging systems used. The opening premise of the FDA guidance acknowledges that general-use X-ray imaging devices typically do not reference specific populations in the indications for use, including pediatrics, and as a result the systems may be used for any size patient. “The adult market for imaging equipment is large and the pediatric market is small,” said Strauss.
Compounding the issue of unspecified indications for use is the fact that, according to Strauss, only about 20 percent of pediatric imaging exams take place in dedicated children’s hospitals. This means that the vast majority of children are imaged in adult hospitals where the imaging systems are not adjusted to specifically meet the imaging needs of children.
“Most X-ray imaging devices in use today can be safely optimized for imaging children, but we’ve heard from healthcare providers that many of these devices lack clear instructions for pediatric use or instructions on how to optimize dose,” said Laurel Burk, Ph.D., biomedical engineer in the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health (CDRH). Burk was the main presenter in a webinar the FDA hosted on the pediatric guidance in January 2018.
Pediatric Indications in 510(k) Submissions
One point the document clarifies is meant to make it easier for X-ray vendors to help their customers adjust the dose for pediatric patients. According to Strauss, in the past when providers have asked for vendor assistance with pediatric dose issues, there has been question of whether such assistance would require the vendor to submit a new FDA 510(k) application for the device in question. On this matter, the November guidance states, “For previously 510(k) cleared X-ray imaging devices, optimization of imaging parameters and provision of pediatric-specific protocols by manufacturers solely at the request of end users generally does not by itself necessitate submission of a new 510(k) submission.”
Strauss said the clarification could help improve the effectiveness of the working relationship between imaging vendors and providers. “When a manufacturer sells a piece of imaging equipment to a dedicated pediatric hospital, it’s understood that will be its primary use and it’s up to the vendor to help the customer fit the equipment to their needs,” he said. Strauss noted that this relationship also works the opposite way — vendors may need to help adult hospitals that image pediatric patients only occasionally.
The FDA guidance does make suggestions for how to enhance new 510(k) submissions for X-ray systems that may have pediatric applications. The 510(k) submission is recommended to include pediatric populations anywhere applicable, according to Burk, including in the following sections:
• Risk assessment — Vendors should consider that potential hazards could be different for patients of different sizes;
• Protocols — Vendors are recommended to include pediatric-appropriate protocols for common procedures adjusted for the patient’s size or weight. The FDA also recommends listing all available pediatric protocols for customers in a downloadable electronic format; and
• Performance testing — According to Burk, performance testing should cover the full indicated patient size range, “which for pediatrics can range from small neonate to older pediatric patients who are similar to an adult size.” She added that if a specific test has a well-understood or linear size dependence, vendors may provide tests for only the smallest and largest patient sizes, with a justification.
Labeling and Instructions for Use
Perhaps the most important new requirement in the November guidance is the inclusion of a pediatric summary section in the X-ray device’s user’s manual. According to Burk, this applies not just to X-ray devices explicitly indicated for pediatric use, but also for those devices “for which significant pediatric use is anticipated.”
The summary should explain any features of the system specifically indicated for pediatric use (including screenshots of the user interface software controls), and include any appropriate pediatric cautions or warnings. The summary should also include the following caution statement: “Use special care when imaging patients outside the typical adult size range.”
Shared Responsibility for Patient Safety
While the guidance is intended for X-ray imaging vendors, Burk noted that the responsibility for pediatric patient safety is shared equally among vendors, providers and parents. Ultimately, she said that all of the risks and benefits of an X-ray should be communicated to the child’s parents to make the final decision. “Instead of repeating numbers, you can explain the different types of possible risks such as cancer, tissue effect and non-radiation risks, and explain if appropriate that the risks are small in comparison with the benefit offered by the exam,” she said.
Burk also noted that although the new guidance only applies to 510(k) submissions going forward, it does not mean older systems should be considered obsolete. “FDA isn’t suggesting the facilities immediately replace their older equipment because as we mentioned imaging professionals can safely use the equipment that they have right now even if it doesn’t have those instructions for use,” she said.



 February 24, 2026
February 24, 2026