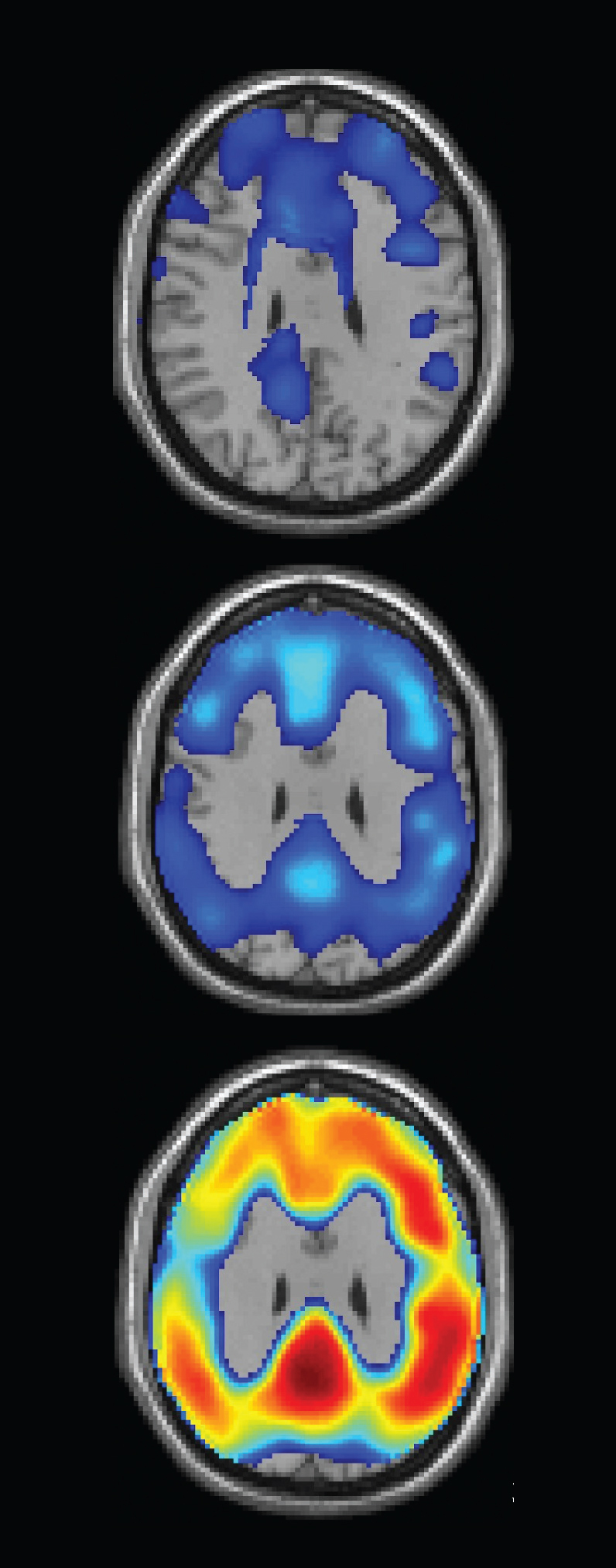
July 12, 2013 — Biopharmaceuticals Inc. announced the first subject has been enrolled in its NAV4694 global, pivotal Phase 3 clinical trial. The trial will assess the safety and efficacy of NAV4694, an investigational positron emission tomography (PET) imaging agent, in detecting cerebral ?-amyloid in end-of-life subjects with and without the diagnosis of dementia. The study will examine the effectiveness of NAV4694 in detecting the presence or absence of ?-amyloid deposition in the brain by directly correlating the PET image findings during life with those of the brain tissue upon autopsy after death.
Currently, only an autopsy can confirm that a person has Alzheimer's disease. “Knowing if a patient has Alzheimer?s pathology in life would lead to improved diagnosis and patient management,” said Frederick Cope, Ph.D., FACN, senior vice president and Chief Scientific Officer at Navidea. “This study, which is designed to provide a direct comparison of patient images collected during life using a ?-amyloid PET imaging agent with postmortem histopathology findings, has the potential to aid the advancement of neurodegenerative disease research and patient care.”
“We are excited to begin this pivotal Phase 3 trial for NAV4694. We believe that ?-amyloid imaging has the potential to play an increasingly important role in clinical practice allowing for earlier diagnosis and therapeutic intervention,” said Connie Reininger, M.D., Ph.D., Navidea’s senior vice president and chief medical officer. “Results from earlier trials using NAV4694 support our conviction that the outstanding performance characteristics of this imaging biomarker, which include favorable sensitivity, specificity, rapid brain uptake and improved image clarity due to low white-matter uptake, position NAV4694 as a true ‘best-in-class’ second generation agent to aid in the diagnosis of Alzheimer’s disease and with the potential to enable diagnosis earlier in the course of disease.”
For more information: www.navidea.com


 February 13, 2026
February 13, 2026 









