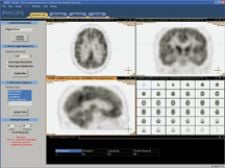
A computer-aided diagnosis system by Philips Medical Systems automatically interprets PET brain scans of patients suspected of having a neurodegenerative disease.
In most cases, neurodegenerative diseases are detected after the patient has already suffered the majority of neural damage. Currently, little exists in the way of science to elucidate the progression of many neurodegenerative diseases. Subsequently, few therapies exist to delay and/or correct neural damage.
However, our understanding of neurodegenerative disease may soon change as clinicians gain greater clarity with the new magnetic resonance (MR) and positron emission tomography (PET) hybrid modality. Siemens Medical Solutions recently demonstrated the world’s first MR-PET system capable of simultaneously imaging the brain. Such a system enables exceptional soft tissue contrast and high specificity of MR together with PET’s excellent sensitivity in assessing physiological and metabolic states.
New insights into the brain
The potential applications of this new technology rest on its ability to provide deeper insight into a variety of neurological disorders like Alzheimer’s, Parkinson’s, epilepsy, depression and schizophrenia. Currently, PET is capable of distinguishing mild cognitive impairment from early-stage Alzheimer’s, however, brain volume changes due to atrophy still go undetected.
Nealie Hartman, clinical marketing manager, MR division, Siemens Medical Solutions, believes that, “[t]he applications will be unlimited especially in neurology and neuro-oncology. There has also been an interest expressed in the psychiatric community.” With the MR-PET scanner, Hartman stated, “You can do all of the functional MR imaging in addition to looking at the uptake of radiotracers. One of the current challenges of functional imaging is replicating that identically every time. That’s the biggest hurdle.” However, with this new technology both MR and PET will enable simultaneous imaging, which overcomes this obstacle. By combining the two technologies, physicians may be able to more accurately diagnose and detect cognitive impairment and atrophy due to the hybrid’s improvement in spatial correlation, while reducing scan time.
MR-PET made compatible
The merger between these two different technologies, MR and PET, involved integrating a PET detector into the bore of a 3.0T scanner and using MR compatible materials. By replacing Photomultiplier tubes (PMT) with Avalanche Photodiode Detector (APD) technology combined with Lutetium-oxyorthosilicate (LSO) crystals, their smaller size and resistance to magnetic fields enabled MR compatibility. Five LSO-APD block detectors from 32 cassettes were arranged axially in Siemens’ Trio MR scanner, creating an axial field-of-view of 19 cm and an inner ring diameter of 35.5 cm. A standard birdcage transmit/receive head coil from Siemens was mounted on the scanner bed and placed inside the PET detector to allow the simultaneous acquisition of PET and MR data.
In the next three to six months, researchers will further evaluate this new technology at five undisclosed beta sites, three in Europe and two in the U.S., where MR-PET units are operating. Although the neurological community sees the promise and potential of this hybrid technology from the first patient data collected at the University of Knoxville at Tennessee, Hartman noted, “I think that we have a lot to learn. Our clinical partners will tell us what is technically relevant. We still don’t know what the final product is going to look like. They may go in and say we have to scatter in a certain way. We may have to refine all of that and incorporate it into our engineering plan and put that data into the final product.” Hartman added, “Depending on what the recommendation is and what we find in the first couple of inserts, we may reassess what is technically relevant to make it as useful and as accurate as possible.”
Weighing the potential cost of this new technology and how it may impact medicine, Hartman indicated, “Initially it will be very expensive. However, when you look at the cost of PET and MR, it would be the same as if you purchased them together. But with the engineering that is involved, it may be even more expensive. Because of the research component and applications, in the next 10 years, it may be something that [smaller facilities] would want. It has such an incredible impact on clinical applications.”
CAD for MR-PET
Another promising new technology for detecting neurodegenerative disease is a computer-aided diagnosis (CAD) technology, currently being tested by Royal Philips Electronics. Together with the University Medical Center Hamburg-Eppendorf (UKE) in Germany, Philips has developed an MR-PET image software tool for the diagnosis of neurodegenerative diseases. This system is a software package that automatically interprets PET brain scans of patients suspected of having a neurodegenerative disease that leads to dementia and combines them with MRI scans for accurate differential diagnosis. This CAD prototype has been optimized to work in combination with Philip’s imaging modalities. However, in principle, the prototype should also work for images obtained from PET and CT scanners from other brands.
The database is composed of only PET images and was developed using a database of normal controls and disease-afflicted patients, which included Alzheimer’s, Lewy-body disease and frontotemporal dementia. The first stage in the CAD process is automatic alignment of FDG-PET brain-scan images to a reference model of the human brain — a process known as ‘elastic registration.’ This not only corrects for the orientation of a particular patient’s head in the scanner, but also for anatomical variation in the brains of different patients. The ‘elastically registered’ FDG-PET image is then compared with a set of reference brain scans that represent typical disease patterns for Alzheimer’s disease, Lewy-body dementia and frontotemporal dementia. Depending on the fit between the patient’s scan and these reference scans, the system provides clinicians with a probable diagnosis.
The CAD system will be clinically evaluated by running it alongside UKE’s existing diagnostic procedures. UKE will apply the system to brain scans in routine clinical patient care. The new CAD system will be compared with visual evaluation by UKE’s expert readers; voxel-based statistical evaluation using a statistical parametric mapping based software package, which was developed in the Nuclear Medicine department at UKE; and the PALZ tool of the PMOD software package, which is an automated method for the discrimination between Alzheimer’s and normal controls.
“In the first evaluation phase we will use the clinical follow-up as the gold standard,” said Steve Klink, senior communication manager, Philips Research. “Patients referred to PET imaging/CAD diagnosis typically have very mild and/or rather unspecified clinical symptoms, which do not allow a reliable diagnosis based on symptoms alone. However, after PET imaging/CAD diagnosis, most patients are further attended by the referring psychiatrist or neurologist. If the patient does have a neurodegenerative disease, the disease will progress and the symptoms will worsen and will become more specific for a neurodegenerative disease. Therefore, it is in many cases possible to make a reliable diagnosis, one year or so after PET/CAD diagnosis. This clinical follow-up diagnosis will be the gold standard in the first phase. We will include only patients with whom a reliable follow-up diagnosis will be available after one year.”
The goal is to improve the accuracy of the diagnosis at an early stage to improve patient outcome. If detected early, patients will be able to take medications earlier, thereby delaying the progression of the disease and the worst effects of dementia. It will also provide pharmaceutical companies and clinicians with a valuable tool for the development and testing of new, potentially curative drugs. The hope is that the CAD software will not only be able to detect disease but will also be able to differentiate diseases.
Ralph Buchert, Ph.D., senior physicist at the Department of Nuclear Medicine, University of Hamburg-Eppendorf, who has been a key developer of this new technology said, “We hope to show that the CAD software will improve the diagnosis, but currently this is a hypothesis. However, I think that this evaluation will be completed successfully. We just need to get hard data.”
Both the CAD technology and the MR/PET scanner are in the research development stage, but both show significant promise in providing better care for patients afflicted with neurodegenerative diseases. <

 March 05, 2026
March 05, 2026 









