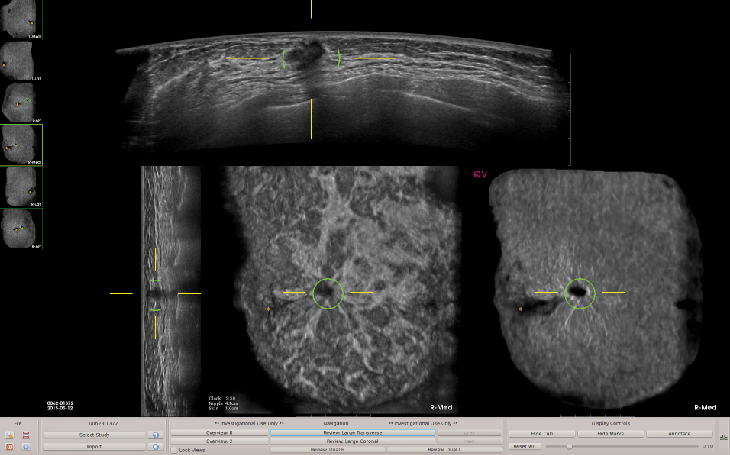
This image depicts ABUS images with QVCAD results.
Imaging Technology News spoke with Bob Foley, vice president of sales and marketing of QView Medical, Inc., about the latest technology in automated breast ultrasound (ABUS). Foley has more than 25 years of experience in medical imaging, with breast cancer detection being his primary focus, and innovation has played a key role. His entry into medical applications was the introduction of Integrated Surgical Systems (Robodoc), the first and only active robot that has performed human surgery. Foley shares his industry insights with us below.
ITN: You have been involved in big data, surgical robotics and now artificial intelligence (AI) computer vision for breast cancer for more than 25 years. There have been key innovations that have occurred in those years designed to improve detection. Over the past 15 years, there has been a tremendous push to digital mammography and tomosynthesis to achieve improved workflow and screening efficiency. What has been the true impact of digital mammography?
Foley: To date, mammography is the only screening modality that has been shown to reduce mortality. It has been the gold standard for breast cancer screening for more than 30 years. Recent improvements, such as the transition from film to full field digital and more recently, to tomosynthesis, have incrementally improved detection and reduced false positives. However, even with these advancements, there are still significant limitations that cannot be overcome with X-ray based imaging, particularly in women with high breast density. Common in younger women, dense breast tissue reduces mammographic sensitivity and increases the risk of getting cancer. As personalized medicine is increasingly being used in the treatment of breast cancer, we must apply the same mentality to screening and diagnosis, where newer, better modalities are available.
ITN: As breast density has become better understood as a risk factor, how has it driven the need for personalized screening?
Foley: There are now 37 states that have passed density inform legislation and we recently saw the passage of federal legislation which directs the U.S. Food and Drug Administration (FDA) to develop effective reporting language for relaying breast density status and ensuring mammography reports delivered to patients and providers contain appropriate information about breast density. This will continue to raise public awareness and give women the ability to ask questions about what other screening tests should be done. There has to be more advice and direction given by the referring physicians and radiologists to women when they receive their mammography results with the breast density assessment — this will be customized and personalized screening.
With improved awareness comes the ability to employ a true multimodality approach to breast cancer detection — and not cling to the one-size-fits-all model that doesn’t work. This includes a combination of screening tools such as mammography, ultrasound and magnetic resonance imaging (MRI) — all of which are available and in clinical practice today. In relationship to breast density seen in more and younger women, it is important we open the discussion of using breast ultrasound to screen at a younger age.
ITN: Why have you been a long-time proponent of breast ultrasound as a screening tool?
Foley: We first became aware of the importance of ultrasound in the mid-1990s when we studied breast cancers from symptomatic patients that were not visible in mammography. Since then, there have been several large-scale clinical studies which demonstrate that breast ultrasound detects cancer where the sensitivity of mammography is limited, especially in dense breasts. One study showed a detection rate of 3.5 cancers per 1,000 mammo-negative patients, and breast ultrasound has been proven to be a very effective supplemental screening modality. And these are small, node-negative cancers that can be treated with positive outcomes, if we can find them early enough. These are the cancers that we want to find — the cancers that we have to find.
Second, with no radiation, ultrasound opens up the screening age to much younger women, perhaps even starting at age 25. This is a solution for asymptomatic patients who may not otherwise think of this as an option because of young age or no family history. And last, women know that ultrasound is safe as many have used it in prenatal stages.
ITN: As you worked on the development of ABUS, what were the capabilities you knew the system had to have in order for it to feasibly support ultrasound screening?
Foley: First and foremost, we were driven to develop a screening tool that could effectively detect lesions in dense tissue. Handheld ultrasound (HHUS) has been used for many years as a breast diagnostic tool. However, operator dependence and variability reduced its appropriateness for population-based screening. The development of an ABUS system had to provide breast imagers with the ability to produce a standard, reproducible view year to year, be free of any operator variability, and seamlessly integrate into the clinical process and workflow. Finally, with patient comfort being an ongoing issue with mammography, we wanted to create a tool that would be comfortable to the patient as well.
ITN: Since being FDA approved in 2012, ABUS has seen measured adoption. Why?
Foley: As with any new modality or disruptive technology that changes doctor-patient care, adoption takes time. ABUS was not only a new way of acquiring breast ultrasound exams, but also represented a new way of looking at screening. Results from several clinical studies have shown that the addition of ABUS to screening mammography results in a significant increase in cancer detection in women with dense breasts. However, the interpretation of ABUS exams, with up to 2,000 images per case, is complex and time consuming, particularly for new users. As ABUS manufacturers have improved system performance and patient comfort features, adoption has continued to ramp up and become a larger part of the personalized screening protocols.
ITN: What challenges with ABUS need to be addressed in order to achieve rapid adoption?
Foley: The next step to accelerate the adoption of ABUS is to improve interpretation efficiency — to be able to read ABUS images in less time, with greater confidence and without any compromise in performance.
Twenty years ago, mammography CAD tools were created to help detect breast cancers earlier — to catch cancers that might otherwise have been missed. We were successful to a point — we did see incremental improvements in early detection. However, the true value of CAD should be to deliver significant reduction in actionable cancers or obvious oversights that are missed.
That is our goal with QVCAD, the first AI CAD system FDA-approved for concurrent reading of ABUS exams, which reduces interpretation time of screening ABUS exams while maintaining diagnostic accuracy. Based on deep-learning algorithms, the AI system is designed to detect suspicious areas of breast tissue that have characteristics similar to breast lesions and highlight suspicious areas to distinguish potentially malignant lesions from normal breast tissue.
The study, “Interpretation time using a concurrent-read computer-aided detection system for automated breast ultrasound in breast cancer screening of women with dense breast tissue,” published recently in the American Journal of Radiology (doi/10.2214/AJR.18.19516), demonstrated that QVCAD reduced reader interpretation time of screening ABUS exams by 33 percent while maintaining diagnostic accuracy. In the first study evaluating reader performance of a concurrent read AI CAD system, University of Chicago researchers compared diagnostic accuracy and interpretation time of screening ABUS for asymptomatic women with dense breast tissue with and without the use of the concurrent-read CAD system.
These results are significant as they show that a concurrent read AI CAD may be an important tool in reducing the learning curve for new ABUS users as well as increasing their confidence in interpreting ABUS exams. These are critical components to continue driving adoption of this very important adjunctive screening modality.
ITN: At RSNA 2018 there were 40 companies touting AI. At RSNA 2019, there will be a separate floor for AI. How do you see AI’s role in healthcare and radiology in the next 10 years?
Foley: Many of the AI applications in healthcare today focus on treatment techniques and patient outcomes. There are only a limited number of those that focus on computer vision. AI, as a concept, has been with us for quite some time. The term was first coined in 1955 by the late John McCarthy, a professor at Stanford. It was introduced into radiology in the 1990s with CAD systems for mammography screening.
Without a doubt, AI, with computer vision, will be vital to the practice of radiology. Imaging systems are improving with more and more images, and higher resolution. Computer systems are more powerful, algorithms are improving and radiologists’ workload is increasing. We are just at the beginning of this paradigm shift.
Related content:
QView Medical Showcases QVCAD for ABUS Exams at RSNA 2018
Artificial Intelligence Helps Detect Breast Cancer and Saves Time
FDA Proposes New Rules for Mammography Reporting and Quality Improvement



 March 06, 2026
March 06, 2026 









