
Dense breast tissue categories include fatty, scattered fibroglandular density, heterogeneously dense and extremely dense.
If you are confused about the conflicting advice surrounding mammography screening guidelines, welcome to the club. When should mammography screening begin? How often is screening necessary? When should screening end? The varying national breast screening guidelines may have both patients and health providers uncertain as to appropriate recommendations. As the screening guideline discussion is one which has found traction in the news and on social media, it may be a challenge to address a patient’s questions about why her own screening protocol differs from what she may be hearing.
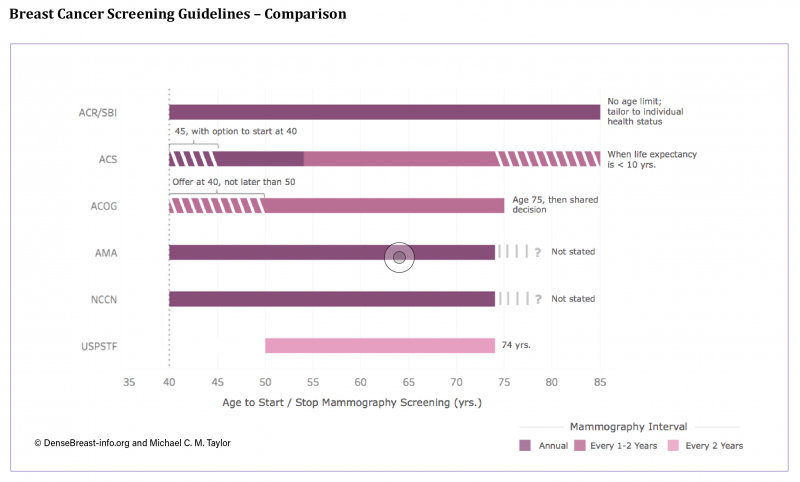
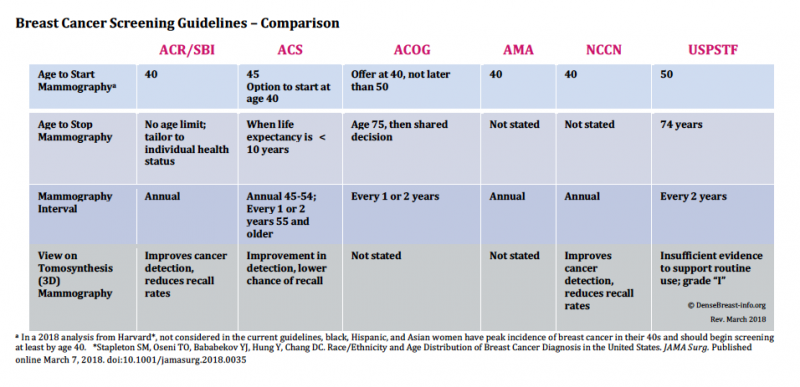
In January 2016, and with much media attention, the U.S. Preventive Service Task Force (USPSTF) issued its final breast cancer screening recommendations. These recommendations differ from those of several national health organizations and have generated confusion and conflicting messaging on the topic within the public arena. Since the USPSTF final guidelines were issued — which recommend screening begin at age 50 and at two-year intervals — the American College of Radiology/Society of Breast Imaging (ACR/SBI), the American Cancer Society (ACS) and the American Congress of Obstetricians and Gynecologists (ACOG) have also updated their own recommendations.
To assist with an analysis of the guideline recommendations, DenseBreast-info.org developed the Breast Cancer Screening Guideline Comparison. Note that the ACS now recommends screening starting at age 45 with an option to start at 40, while ACOG recommends offering screening starting at age 40 but not later than 50. The ACR/SBI, American Medical Association (AMA) and National Comprehensive Cancer Network (NCCN) continue to recommend screening beginning at age 40 and continuing at an annual interval. The ACS suggests mammography interval annually for ages 45-54, and every one to two years at age 55 and older, while ACOG suggests every one to two years without age qualifiers.
The age to stop mammography also differs among guidelines and varies from a specific age cutoff (USPSTF suggests 74 years of age, ACOG suggests age 75 and then shared decision making), to stopping when life expectancy is less than 10 years (ACS), to no specified age limit with recommendation tailored to the individual patient’s health status (ACR/SBI). The AMA and NCCN do not state an age to stop mammography screening.
In light of the growing availability and utilization of 3-D mammography (also known as tomosynthesis), several guidelines also include their view on the technology’s benefits, and these also differ. The ACR/SBI, ACS and NCCN find it an “improvement” over 2-D mammography in terms of detection and recall. Because the USPSTF only considers data from randomized trials with mortality as an endpoint, and there are no such trials of any breast imaging other than mammography, USPSTF’s position is that there is still “insufficient evidence to support routine use.” The ACOG and the AMA do not state positions on 3-D.
Given the disparity in the guidelines, it can be a challenge to explain why and what the most appropriate screening protocol is for a patient. DenseBreast-info.org believes that patients should start their screening mammograms before age 45 for the following reasons:
• The entire reason we screen for breast cancer is to find it early, when most treatable and survivable.
• Breast cancer is the No. 1 cause of death in women aged 35 to 54 years.
• Mammography has been proven to reduce deaths due to breast cancer in women screened beginning at age 40.
• 25 percent of all years of life lost to breast cancer occur in women diagnosed before the age of 45.
• Women at “high risk” for breast cancer due to known or suspected disease-causing mutation (such as BRCA1 or BRCA2) should begin screening at least by age 30, to include magnetic resonance imaging (MRI).
The American College of Radiology also recommends annual MRI in addition to mammography in women determined to be at “higher than average risk,” which includes all women diagnosed with breast cancer at/by age 50 and women diagnosed at any age with a personal history of breast cancer and dense breasts.1
What about false positives?
• About 10 percent of women having a screening mammogram will be called back for extra testing or views. This is normal. Among women called back, 95 percent do not have cancer. If a needle biopsy is necessary, even that is a simple test.
• The newer technique of 3-D mammography (tomosynthesis) is better able to show cancer and results in fewer callbacks for extra testing.
What about screening in dense breasts?
• Younger women are more likely to have dense breast tissue, which can hide cancer on mammography.
• In women who have breasts categorized as “dense” (heterogeneously dense or extremely dense), adding screening ultrasound (if the woman is not a high risk) after a mammogram (2-D or 3-D) can help find more breast cancers. Because ultrasound detects more areas that need follow-up, there is more to check. Ultrasound also increases the chance of needing a needle biopsy to determine if something detected is cancerous or not.
Reference
JoAnn Pushkin is executive director of DenseBreast-info, Inc. For more information on breast screening, risk factors and dense breasts, visit DenseBreast-info.org.
© DenseBreast-info Inc. and JoAnn Pushkin



 March 05, 2026
March 05, 2026 









