Results from a recent prospective trial found the Wide Area Transepithelial Sampling with 3D Tissue Analysis (WATS3D) device increases the detection of both Barrett’s esophagus and esophageal dysplasia by more than 80 percent. Study results from the multicenter trial of more than 4,000 patients were published in the latest issue of United European Gastroenterology Journal and featured in the American Society for Gastrointestinal Endoscopy (ASGE)’s Scope Tech Talk video series.
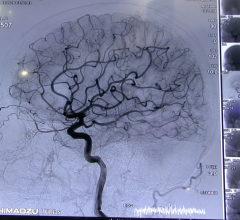
Revised guidelines for the nonsurgical, image-guided interventional treatment of acute ischemic stroke will ensure patients receive the right treatment at the right time. The updated guidelines, endorsed by the Society of Interventional Radiology (SIR) and 11 other medical societies, were published in the April edition of SIR’s peer-reviewed journal, the Journal of Vascular and Interventional Radiology (JVIR).
April 2, 2018 – The Canadian Nuclear Safety Commission (CNSC) announced March 29 that it renewed Canadian Nuclear ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
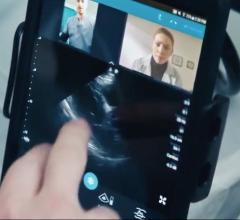
Philips in partnership with Innovative Imaging Technologies (IIT) announced an industry-first integrated tele-ultrasound solution on Philips’ Lumify portable ultrasound system powered by IIT’s Reacts collaborative platform. This innovation connects clinicians around the globe in real time by turning a compatible smart device into an integrated tele-ultrasound solution, combining two-way audio-visual calls with live ultrasound streaming.
The Marcus Foundation has donated $15 million to establish the Marcus Stroke Network, a coordinated and collaborative effort among three healthcare systems and the American Heart Association/American Stroke Association (AHA/ASA). The collaborative will work together to help reduce stroke disability and death rates in the Southeastern United States.
Canvys, a Division of Richardson Electronics Ltd., recently enhanced its 4K Ultra HD custom display series of high-brightness monitors with two new screen sizes. The new 27-inch and 32-inch ultra HD monitors are designed to deliver high quality images with top work performance. In addition, Canvys is now offering optical bonding as a custom option for this series up to 32 inches.
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Clarius Mobile Health is introducing its Clarius Medical School Award Program. Under that program, Clarius will grant over $200,000 worth of Clarius Ultrasound Imaging scanners to selected medical schools after applications are reviewed by an independent clinical panel.
NTT Data Services this week announced a partnership with DataFirst Inc. to deliver clinical artificial intelligence (AI) that will help healthcare organizations improve quality and decrease the cost of patient care.
March 29, 2018 — The Aquilion One/Genesis Edition computed tomography (CT) system from Canon Medical Systems gives ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
The American College of Radiology (ACR) released new breast cancer screening guidelines recommending all women, especially black women and those of Ashkenazi Jewish descent, should undergo risk assessment by age 30. Assessing risk at this age would allow women at higher risk to be identified and begin earlier and more aggressive screening for breast cancer. In a separate recent analysis[i] from Harvard, black, Hispanic and Asian women have peak incidence of breast cancer in their 40s and should begin screening at least by age 40.
Canon Medical Systems USA Inc. received U.S. Food and Drug Administration (FDA) approval for a robust suite of quantitative tools for the Aplio i800 ultrasound system to assess the spectrum of liver disease. The new tools may be helpful in the assessment of liver diseases such as steatosis, Non-alcoholic fatty liver disease (NAFLD) and/or non-alcoholic steatohepatitis (NASH), which are characterized by elevated levels of fat accumulation in the liver.
Hologic Inc. announced that Clarity HD high-resolution 3-D imaging and Intelligent 2-D imaging technology have received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) and are now available on the 3Dimensions breast tomosynthesis system. With these innovations, the system now provides higher resolution 3-D images for radiologists, enhanced workflow for technologists, and a more comfortable mammography experience with low-dose options for patients.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Imaging Technology News has been recognized with an award nomination from the Jesse H. Neal Awards as a finalist for the ...
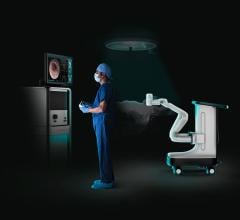
Physicians, trainees and even laypeople can now stand right beside an expert radiologist as he performs one of the most difficult medical procedures of its kind – in virtual reality.
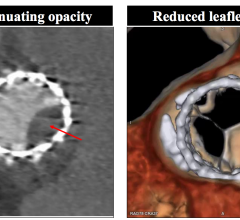
March 27, 2018 — Edwards Lifesciences Corp. announced that enrollment is complete in the computed tomography (CT) ...
March 27, 2018 — While there are many treatment options for men with prostate cancer, a recent national study published ...
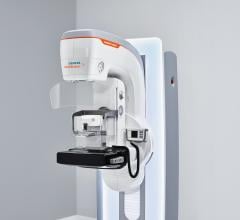
March 27, 2018 — The U.S. Food and Drug Administration (FDA) has cleared Siemens Healthineers’ Mammomat Revelation ...
Medical school students receive little formal instruction in radiation oncology, a Loyola study has found.
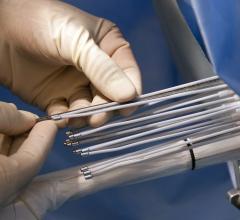
March 26, 2018 — Singular Medical Technologies (SMT), an emerging technology company focused on high-performance ...
March 26, 2018 — Auris Health Inc. announced U.S. Food and Drug Administration (FDA) clearance of the company’s Monarch ...

 April 02, 2018
April 02, 2018