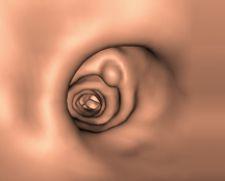
Cook Medical's FDA-cleared Evolution Controlled Release Esophageal Stent System is a stent designed to improve the quality of life for patients with esophageal cancer.
June 10, 2008 - The use of ultrasound contrast agents during stress echocardiograms is safe, according to results ...
June 10, 2008 – The design of the University of Chicago Medical Center’s New Hospital Pavilion offers flexibility by ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
The future is looking bright for virtual colonoscopy (CTC) after encouraging news came out that the Centers for Medicare ...
Candelis received 510(k) marketing clearance for its ImageGrid Mammography Web Viewer and ImageGrid Radiology Web Viewer ...
Acusphere has submitted a New Drug Application (NDA) to the FDA for approval to market Imagify (Perflubutane Polymer Microspheres for Injectable Suspension), an ultrasound imaging agent for the detection of coronary artery disease, which could prove as accurate as nuclear stress testing.
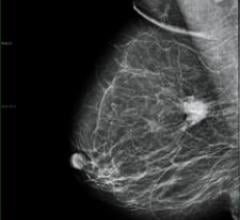
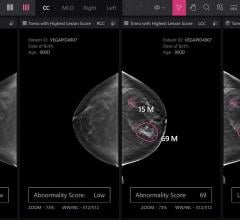
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
June 9, 2008 - Siemens Medical Solutions gained FDA 510(k) market clearance for the SOMATOM Definition AS, what is said ...
Toshiba Teli America introduced the T24MSA001-MD medical-grade 24-inch LCD color widescreen monitor with full HD (1,080 ...
ProHance is a macrocyclic, nonionic, gadolinium-based magnetic resonance imaging (MRI) contrast agent designed for use in MRI in adults and children over two years of age to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine and associated tissues. ProHance is also indicated for use in MRI in adults to visualize lesions in the head and neck.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
June 9, 2008 – American TeleCare Inc. (ATI) released Quick Notes as a new feature of its inLife/LifeView Telehealth ...
The FDA granted tentative approval for Covidien’s Abbreviated New Drug Application (ANDA) for its Kit for the Preparation of Technetium Tc 99m Sestamibi Injection. Covidien’s tentatively approved product is a generic of Cardiolite, which is a myocardial perfusion imaging agent used for detecting coronary artery disease.
June 9, 2008 - The FDA cleared Mindray’s DC-3 color ultrasound imaging system, equipped with extensive applications in ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
June 9, 2008 - Cardinal Health today launched a new system that leverages the company’s dispensing technologies to ...
With radiologists in short supply, the need for teleradiology services continues to grow and the market has responded in ...
The FDA recently approved Covidien’s contrast delivery system with radiofrequency identification (RFID) technology ...
The EmpowerMR injector system has multiple features created to cope with the problem of electrical interference in the ...
June 9, 2008 - Hologic Inc. said today that it has signed a definitive agreement to acquire Third Wave Technologies Inc ...
GE received FDA clearance for its new Signa MR750 3.0T scanner that delivers up to 60 percent additional anatomical ...
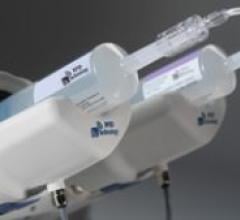
The ACIST CVi Contrast Delivery System provides one injection system for all cardiac and vascular angiography procedures. The variable-rate ACIST CVi system aims to provide ease of operation for clinicians, while obtaining quality images with less contrast used and delivered to the patient.
June 9, 2008 - Curlin Medical was recently awarded a three-year contract to provide its advanced pain management-local ...

 June 09, 2008
June 09, 2008