MediNotes Corp.
Technology
Search by Company Name, Product Category, Product Name or by any combination of the three using the search boxes below.
The FDA cleared St.
Amicas demonstrated Vision Series PACS Version 5.5 at DHIMS 2008.
The FDA 510(k) cleared the new Fuji DR (FDR) system, Unity SpeedSuite, a single detector…
Carestream Health showcased new capabilities for its Carestream RIS and PACS platforms.
Siemens Medical Solutions received FDA 510(k) marketing clearance for the sale and distribution…
The latest offering of NovaPACS and NovaRIS 7.0 offers tighter integration between the systems,…
Hitachi’s HI VISION 5500 ultrasound system, found in Radiology departments and diagnostic…
ScImage gained FDA clearance for primary diagnostic review of mammographic images using its…
Acuo Technologies showcased the 6.0 version of its DICOM Services Grid solution, a distributed…
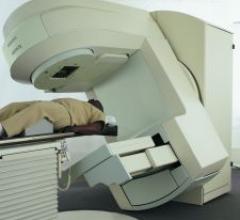
Nucletron released three new products designed to improve brachytherapy practice.
Toshiba America Medical Systems received FDA clearance for the new open-bore 1.5T Vantage Titan…
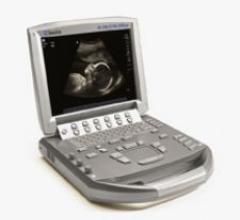
SonoSite Inc.
Philips’ Tumor Localization (Tumor LOC) application reportedly allows radiation oncology…
The Vision 2008 upgrades to the Philips iU22 aim to bring expanded volumetric capabilities, new…
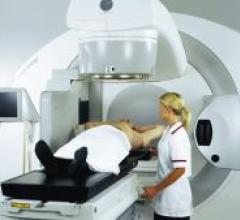
The FDA cleared Siemens’ new ARTISTE Solution, a linear accelerator engineered for Adaptive…
The FDA gave market approval to the Medical Office Suite of Products, which aims to address the…
Based on Zone Sonography technology, the z.one ultra system now features advanced software,…
Elekta’s Synergy, a Modulated and Image Guided Radiation Therapy (IMRT and IGRT) system is…

 April 08, 2008
April 08, 2008