Virtual Phantoms Inc. announced the release of VirtualDoseIR, a tool for assessing organ dose…
Technology
Search by Company Name, Product Category, Product Name or by any combination of the three using the search boxes below.
GE Healthcare announced Signa Premier, a new wide bore 3.0T magnetic resonance imaging (MRI)…
August 3, 2017 – Fujifilm Medical Systems U.S.A. Inc.
July 31, 2017 — Accuray Inc. announced it has received 510(k) clearance from the U.S.
July 27, 2017 — CorTechs Labs recently announced that Hitachi 1.2T Oasis, 1.5T Echelon Oval and…
Agfa HealthCare announced it has received U.S. Food and Drug Administration (FDA) 510(k)…
July 24, 2017 — TomTec Zero is the latest addition to the TomTec portfolio.
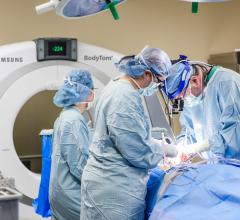
Samsung announced U.S. Food and Drug Administration (FDA) approval of the BodyTom Elite, an…
The U.S. Food and Drug Administration (FDA) has cleared the first magnetic resonance imaging (…
July 13, 2017 — Vital Images Inc.
Varian Medical Systems has received U.S. Food and Drug Administration (FDA) 510(k) clearance for…
Philips announced it has received 510(k) clearance from the U.S. Food and Drug Administration (…
June 27, 2017 — Toshiba Medical, a Canon Group company, introduced its newest…
Unfors RaySafe recently introduced the RaySafe Pro-Slit Phantom and the RaySafe Pro-Stand.
June 16, 2017 — The U.S.
June 1, 2017 — QUBYX Software Technologies Inc. received U.S.
MIM Software Inc. recently announced significant updates to its MIM Encore solution for viewing…
Intelerad Medical Systems launched CosmosOne, a living medical archive that centralizes all…
NucleusHealth has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its…
Image Diagnostics Inc. (IDI) recently unveiled the Uni Diagnost, the first integrated…


 August 07, 2017
August 07, 2017