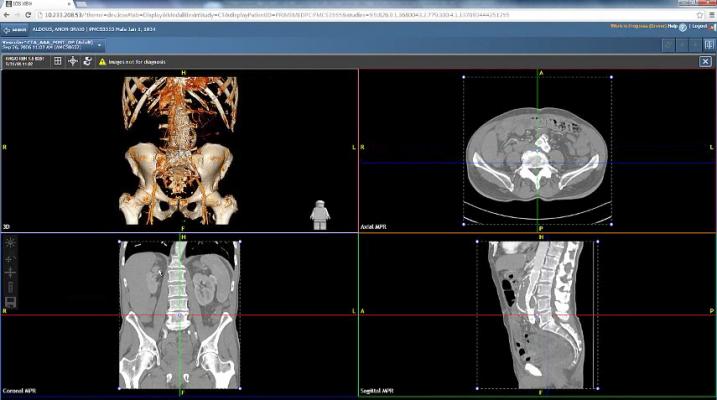
July 27, 2017 — Agfa HealthCare announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Xero Xtend functionalities of the Enterprise Imaging zero-footprint viewer. Xero Xtend adds 3-D processing and advanced clinical applications to the Xero Viewer. It is now FDA-cleared for diagnostic viewing on desktop devices; Xero Xtend is not FDA-cleared for mobile viewing at this time.
The Xero Viewer provides secure access to DICOM and non-DICOM imaging data from different departments and multiple sources, in one view, to anyone inside and outside of the hospital who is subject to defined security policies and access rights.
Images from the Agfa HealthCare Enterprise Imaging IT platform or third-party vendor neutral archives (VNAs) or picture archiving and communication systems (PACS) can be seen securely in one display.
Xero Xtend is an optional feature set for the Xero Viewer. It includes:
- Maximum intensity projection (MIP), multiplanar reconstruction (MPR) and 3-D visualization tools;
- Orthopedic tools (center line, Cobb Angle, Hip-Knee-Ankle Angle, etc.);
- Mammography tools (including displays and tools for optimal viewing); and
- Nuclear medicine tools (color highlighting palettes stored as window level presets).
Agfa HealthCare has also expanded the types of modalities that can be used for diagnostic review and reporting in Xero. Uncompressed or non-lossy compressed images intended "for presentation" may be used for diagnosis or screening when viewed on monitors that have been cleared by the FDA.
In addition, the Full Fidelity Mobile functionality (mobile diagnostic review and analysis on an iPad), which received FDA 510(k) clearance in 2015, now includes electrocardiogram (ECG) images and reports.
The Xero Viewer recently received top honors from KLAS, with its 2017 Category Leader Award. This is the first year the healthcare quality research organization has included a Universal Image Viewer category. The Category Leader designation is reserved for vendor solutions that lead select market segments in which at least two products meet a minimum level of KLAS confidence. According to the latest KLAS Performance Report, Agfa HealthCare is one of two vendors offering universal viewers that are fast and reliable, and noted for continually improving their solutions.
For more information: www.agfahealthcare.com


 February 16, 2026
February 16, 2026 








