Dec. 4, 2024 – Konica Minolta Healthcare Americas and Gleamer have announced a strategic partnership to help radiologists further enhance the quality of care and optimize workflows in musculoskeletal (MSK) digital radiography (DR) applications. Konica Minolta will offer Gleamer’s BoneView solution with its DR product portfolio, including the KDR Advanced U-Arm, KDR Flex Overhead X-ray System and mKDR Xpress Mobile X-ray, making the AI-based solution available to customers in the Americas.
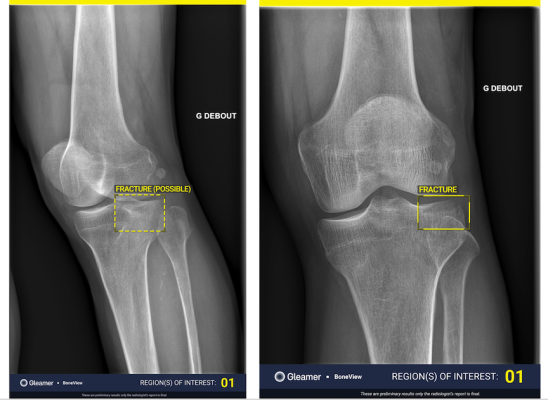
BoneView is a clinical decision support AI-based software solution designed to efficiently identify bone fractures by highlighting areas of interest on an X-ray image, assisting both radiologists and clinicians in their daily practice. This FDA-cleared, computer assisted detection solution (CADe) has been trained on tens of thousands of radiographic images and generated clinical evidence through 80 clinical studies and nearly 25 peer-reviewed scientific publications. It is the only such application that is cleared for use in some pediatric anatomical areas.1 BoneView efficiently highlights fractures and provides confidence levels, such as highlighting 90% as probable. This capability helps boost diagnostic accuracy, reduce radiologist reading times and improve patient care pathways.
Konica Minolta will offer the cloud-based BoneView solution as a subscription-based service to its customers. Designed around patients for clinical efficiency, Konica Minolta’s DR systems feature an array of design innovations to deliver superior image quality and optimize workflow to expedite the diagnostic process. The AeroDR family of high-performance wireless flat panel detectors deliver exceptional image quality, including a High-Definition option for excellent detail at 100u and a High Dynamic Range option that provides a 200u resolution to help visualize depth and definition of complex soft tissue/bone structures.
“We are very excited to partner with Gleamer and provide BoneView AI to our DR customers in the Americas,” says Kirsten Doerfert, Executive Vice President of Marketing, Konica Minolta Healthcare. “BoneView is an impressive application for detecting bone fractures in X-ray images. Our choice to partner with Gleamer was based in part on Gleamer's commitment to clinical evidence, giving customers a high level of confidence in the application’s results. This excellent diagnostic support tool complements our portfolio of X-ray systems and software solutions for our customers.”
Nicolas Jirikoff, Chief Business Officer of Gleamer, adds, “Our partnership with Konica Minolta marks another step forward in Gleamer's international expansion, bringing us closer to our goal of making our AI Copilot accessible to clinicians and patients worldwide. By combining our proven AI solutions in radiology with Konica Minolta’s expertise as a recognized leader in DR systems, this collaboration will help advance precision medicine across the US and raise the standard of patient care.”
For more information on Konica Minolta Healthcare Americas, please visit https://healthcare.konicaminolta.us. Additional information on Gleamer is available at www.gleamer.ai.
- Please refer to 510(k) K222176 for complete indications for use: https://www.accessdata.fda.gov/cdrh_docs/pdf22/K222176.pdf.


 March 06, 2026
March 06, 2026 









