April 11, 2024 — Prelude Corporation (PreludeDx), a leader in precision diagnostics for early-stage breast cancer, presented data on the use of DCISionRT to identify a subset of patients with high-risk clinicopathology who had a low 10-year risk of in-breast recurrence (IBR) and no significant benefit from radiation therapy (RT). In contrast, patients who had both high risk DCISionRT and high-risk clinicopathology had high 10-year IBR rates and significant RT benefit. The data was presented at the 25th American Society of Breast Surgeons (ASBrS) Annual Meeting, held on April 10 – 14, 2024 at the Orlando World Center Marriott.
“Breast conserving surgery (BCS) followed by RT is considered standard management of DCIS patients. However, we now realize that not all DCIS patients will benefit from RT and there is a growing need to safely de-escalate treatment,” said Julie Margenthaler, MD, FACS, Professor of Surgery, Washington University School of Medicine; Surgeon, Siteman Cancer Center; and first author of the study. “DCISionRT is a valuable clinical tool that enables physicians to help identify which DCIS patients are most likely to benefit from RT based on the patient’s individual tumor biology and can help reduce over- or under-treatment.”
Dan Forche, President and CEO of PreludeDx stated “the data presented at ASBrS further demonstrates the utility of DCISionRT to enable patients and their physicians to make a more informed treatment decision compared to using clinicopathology alone.”
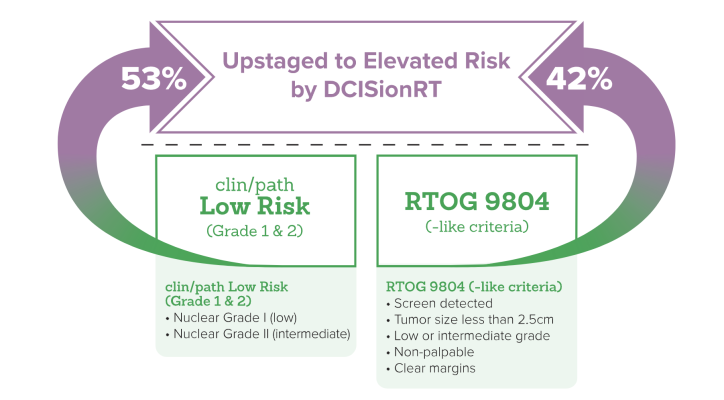
The presentation, ‘Limitations in Utilizing Clinicopathologic Factors Alone in Identifying Patients with DCIS Who Benefit from Radiotherapy after Breast-Conserving Surgery’, showed that about one-third of patients with high-risk clinicopathology who were reclassified into the DCISionRT low-risk group had a 10-year IBR risk of ~6% after BCS and no significant RT benefit.
For more information: www.preludedx.com
Related content:
American Medical Association Grants PreludeDx a CPT PLA Code for Proprietary DCISionRT Test
PreludeDx Presents DCIS Precision Medicine Study Results at ASTRO 2021 Annual Meeting


 February 11, 2026
February 11, 2026 









