November 12, 2021 — Prelude Corporation (PreludeDx), a leader in molecular diagnostics and precision medicine for early-stage breast cancer, announced that the American Medical Association (AMA) has issued a new, dedicated current procedural terminology (CPT) proprietary laboratory analyses (PLA) code for DCISionRT, the only test available to predict personalized recurrence risk and radiation therapy benefit for patients with ductal carcinoma in situ (DCIS). The new code, 0295U, will become effective Jan. 1, 2022, and is specific to the DCISionRT test.
PLA codes are an addition to the CPT code set approved by the American Medical Association CPT Editorial Panel. PLA codes are alpha-numeric CPT codes with a corresponding descriptor for labs that want to more specifically identify their test.
“We are extremely pleased that the AMA has approved a unique CPT PLA code for our DCISionRT test,” said Dan Forche, president and CEO of PreludeDx. “This is another important milestone for our company. The award of this code further validates the enhanced value that DCISionRT provides physicians and their DCIS patients and is an important step in our ongoing efforts to secure broader reimbursement for patients.”
About DCISionRT for breast DCIS
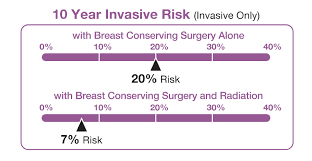
DCISionRT is the only risk assessment test for patients with ductal carcinoma in situ (DCIS) that predicts radiation therapy benefit. Patients with DCIS have cancerous cells lining the milk ducts of the breast, but they have not spread into surrounding breast tissue. In the U.S., over 60,000 women are newly diagnosed with DCIS each year. DCISionRT, developed by PreludeDx on technology licensed from the University of California San Francisco, and built on research that began with funding from the National Cancer Institute, enables physicians to better understand the biology of DCIS. DCISionRT combines the latest innovations in molecular biology with risk-based assessment scores to assess a woman’s individual tumor biology along with other pathologic risk factors and provide a personalized recurrence risk. The test provides a Decision Score that identifies a woman’s risk as low or elevated. DCISionRT’s intelligent reporting provides a woman’s recurrence risk after breast conserving surgery alone and with the addition of radiation therapy. In turn, this new information may help patients and their physicians to make more informed treatment decisions.
For more information: https://preludedx.com/


 March 06, 2026
March 06, 2026 









