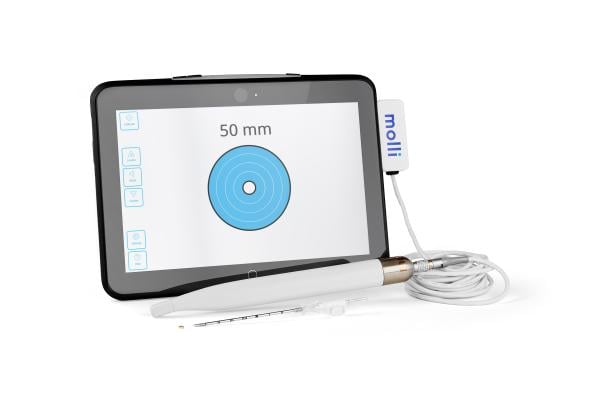
August 19, 2022 — The Christ Hospital Health Network, known for providing the best, most compassionate care for its community, is the first in Ohio to adopt Molli Surgical’s wire-free and radiation-free breast cancer localization technology: Molli. The Molli device features the smallest localization marker on the market that precisely marks where tumors are located prior to breast cancer surgery, helping the surgeon remove them more efficiently and improving the patient’s overall experience.
The Christ Hospital Health Network’s adoption of this technology reflects its commitment to doing everything it takes for patients, such as driving innovations like wire-free localization that improve the patient experience. Going forward, Molli Surgical will be the provider of wire-free, radiation-free technology for breast surgery.
"Marking the tumor for those needing breast cancer surgery can be a difficult experience for the patient — typically using a wire on the same day of surgery. Molli decouples localization from surgery, providing more flexibility for the patient,” says Jennifer B. Manders, MD. “Plus, the device is easy-to-use, efficient, and extremely precise. We chose Molli after testing other wire-free and radiation-free devices as it had proven to deliver superior results and benefits for the patient, the radiologist, and the surgeon.”
“Like ourselves, the team at The Christ Hospital Health Network is dedicated to reimagining what healthcare can be and truly understanding patient-first care,” said Fazila Seker, Ph.D., President, and CEO of Molli Surgical Inc. “We are committed to helping them create more efficiencies in their radiology and surgery departments and deliver exceptional outcomes and experiences for their patients.”
Molli Surgical’s FDA-cleared Molli allows surgeons to pinpoint cancerous tissue for faster, more accurate, and less painful tumor removal, offering an improved experience and better cosmetic outcomes for breast cancer patients, as well as patients living with different types of cancers. The wire-free technology includes the Molli Marker, about the size of a sesame seed, that can be detected using the Molli Wand and the Molli Tablet.
For more information: mollisurgical.com


 February 06, 2026
February 06, 2026 









