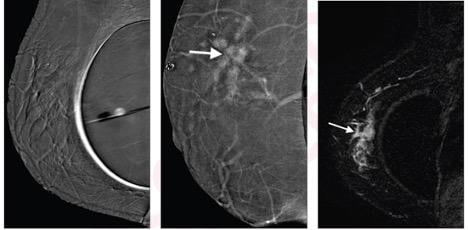
Left to right: Subtraction right mediolateral oblique (MLO) CEM was non-diagnostic because of artifact, potentially due to motion misregistration from extended exposure time; subtraction right MLO implant displaced CEM image shows 5.8 cm enhancing mass (arrow); contrast-enhanced MRI sagittal subtraction image shows concordant mass (arrow).
March 18, 2021 — According to ARRS’ American Journal of Roentgenology (AJR), contrast-enhanced mammography (CEM) showed concordance with magnetic resonance imaging (MRI) in women with newly diagnosed breast cancer and breast augmentation.
Noting that CEM has not been investigated in women with breast augmentation, Molly Carnahan and her Mayo Clinic team in Phoenix, AZ, concluded, “the findings suggest a possible role of CEM for staging in women with breast augmentation and contraindication or limited access to MRI.”
From an institutional database of 2,215 women who underwent CEM between January 2015 and March 2020, the researchers identified breast implants in 67 women: 42 without corresponding MRI, 3 without breast cancer, 1 with axillary disease only, and 6 with neoadjuvant chemotherapy before CEM or MRI — leaving a final sample of 17 women (mean age 52 years; 6 with non-dense breasts, 11 with dense breasts).
The index cancer histology was invasive ductal carcinoma (IDC) in 15 (88%) women, invasive lobular carcinoma (ILC) in 1 (6%), and ductal carcinoma in situ in 1 (6%). Median index cancer size was 2 cm, and 2 (12%) index cancers were mammographically occult. Ultimately, CEM and MRI were concordant for the index cancer in all 17 women.
Six additional lesions were demonstrated by CEM and confirmed by MRI in 6 (35%) women: 3 multifocal, 1 multicentric, 2 contralateral. Two of these lesions revealed malignant histopathology: 1 IDC, 1 ILC.
“MRI did not identify any additional cancers not identified on CEM,” the authors of this AJR article added.
For more information: www.arrs.org


 February 18, 2026
February 18, 2026 









