June 24, 2019 — Artificial intelligence (AI) imaging solution form ClariPi Inc. has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its AI-based computed tomography (CT) denoising technology, ClariCT.AI.
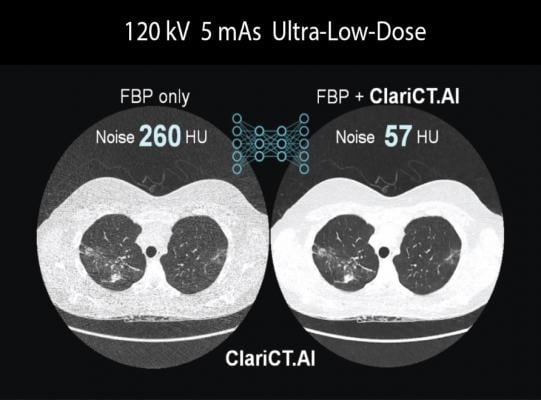
First debuted at the 2018 Radiological Society of North America annual meeting (RSNA 2018), ClariCT.AI uses a deep convolutional neural network, trained to work in a vendor-agnostic way, to reduce noise and enhance image clarity for low-dose and ultra-low-dose DICOM CT images. Trained with more than 1 million patient images containing varying degrees of noise for different body parts, its Clarity Engine separates image noise selectively while enhancing underlying structures, thus providing clarity-restored images.
ClariPi believes that the clarity-restored images have the potential to improve reading confidence of radiologists, as well as to enable accurate analysis by computer-aided solutions for various imaging applications, especially for low-dose lung cancer screening CT.
ClariPi will be showing the ClariCT.AI at the 2019 Society for Imaging Informatics in Medicine annual meeting (SIIM19), June 26-28 in Aurora, Colo.
For more information: www.claripi.com


 February 09, 2026
February 09, 2026 









