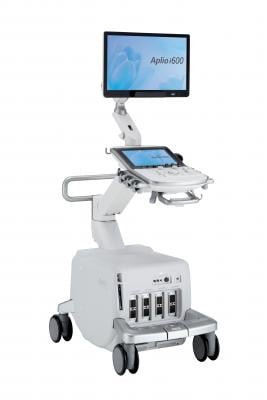
March 26, 2018 — Healthcare professionals seeking a cost-effective imaging solution without compromising image quality can now leverage the newly U.S. Food and Drug Administration (FDA)-cleared Aplio i600 ultrasound system from Canon Medical Systems USA Inc. Combined with a full host of easy-to-use workflow tools and performance features, the Aplio i600 improves diagnostic confidence and enables clinicians to deliver quick, reliable exams, according to Canon.
The Aplio i600 provides intuitive ergonomics to help boost productivity during routine and complex exams thanks to its small and light iSense design, which makes it easy for clinicians to adjust the console to virtually any scanning position. The newly FDA-cleared system also offers an image-guided user interface to visually guide the clinician through the exam, simplifying system operation and helping improve efficiency. To help healthcare providers give more confident diagnoses, this premium performance system features iPerformance imaging technology that reduces clutter, strengthens signal and improves visualization. The Aplio i600 uses the same transducers from the current Aplio Platinum Series, making migration to this new system even easier.
Canon Medical Systems is showcasing the new Aplio i600 ultrasound at this year’s American Institute of Ultrasound in Medicine (AIUM) 2018 annual meeting in New York, March 24 – 28, 2018.
For more information: www.us.medical.canon
Related AIUM Content
Samsung Unveils New RS85A Ultrasound System at AIUM 2018
SonoSim Launches Cloud-Based OB-GYN Ultrasound Training Modules


 February 05, 2026
February 05, 2026 









