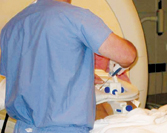
A radiologist teams up with a urologist to perform biopsies using new devices that help diagnose prostate cancer.
Radiologist John Feller, M.D., medical director of Desert Medical Imaging in Indian Wells, Calif., and local urologists have joined forces to test a promising new way to detect and diagnose prostate disease.
When a traditional transrectal ultrasound (TRUS) biopsy proves negative for the presence of disease, yet a patient’ s prostate-specific antigen (PSA) levels continue to rise, magnetic resonance imaging’s (MRI) excellent soft-tissue imaging quality may be the answer to a difficult diagnosis.
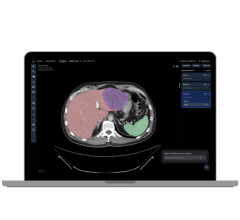
DynaCAD for prostate and DynaTRIM, both part of Invivo’s prostate clinical solution, give physicians the tools to perform real-time analysis of prostate MRI studies and the support for an MRI-guided prostate biopsy. Initial experiences by Feller have shown positive results. The ability to identify metabolically active areas that look suspicious for prostate disease has improved.
A Simple, Targeted Procedure
Following a diagnostic MRI to confirm the presence of suspicious tumor(s), the patient is placed on the scanner in a prone position and the MR-compatible biopsy device, DynaTRIM, is inserted into the rectum.
“The DynaTRIM needle guide is much smaller (approximately one-quarter the circumference) than the endorectal probe used for TRUS biopsies,” comments Feller, “and is so well tolerated that it seems to preclude the need for local anesthetic.”
A new scan confirms the guide’s position, and Feller and the urologist then plan the biopsy. “Using the DynaCAD’s DynaLOC localization software, this is really very simple,” says Feller. “There are three different coordinates — a couple of degree measurements — and length measurements that are quickly generated by the software.”
The DynaTRIM is adjusted and the urologist steps up to perform the actual biopsies. With this targeted approach, only a few core samples are required.
The Proof is in the Results
For patient George LaVie, the MRI-guided prostate biopsy confirmed what his TRUS biopsy could not. Feller notes, “You’d never have been able to hit this with a needle under ultrasound guidance because it was up behind some bones in the pelvis, called the pubic symphysis.
“In LaVie’s case,” he continues, “we took cores from just four different locations, plus a second core from one location by angulating the needle guide a couple of degrees.”
Pathology results confirmed the presence of cancer and LaVie underwent a successful prostatectomy.
Feller is a firm believer in the potential of this new technology. “Many times, when new technology like this is rolled out, it is very complicated and difficult to operate. This isn’t anything like that. This can be done in an outpatient imaging center where the key ingredient is teamwork between the urologist and radiologist,” he explained.
Case study supplied by Invivo Corp.


 February 11, 2026
February 11, 2026