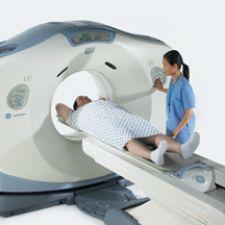
GE Healthcare has received clearance from the FDA for the new wide bore computed tomography (CT) system. This new technology is a 16-slice wide bore CT system, and will be available in two configurations. The LightSpeed RT16 enables advanced imaging for radiation therapy planning, and the LightSpeed Xtra is a 16-slice CT scanner designed for radiology needs such as trauma, interventional and bariatric procedures.


 March 03, 2026
March 03, 2026 









