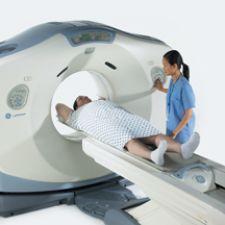
GE Healthcare has received clearance from the FDA for the new wide bore computed tomography (CT) system. This new technology is a 16-slice wide bore CT system, and will be available in two configurations. The LightSpeed RT16 enables advanced imaging for radiation therapy planning, and the LightSpeed Xtra is a 16-slice CT scanner designed for radiology needs such as trauma, interventional and bariatric procedures.
The LightSpeed RT16 is positioned to provide resolution, power and speed to meet extremely challenging clinical needs. The LightSpeed Xtra has gained clearance by the FDA for imaging of obese and morbidly obese patients. LightSpeed Xtra provides productivity capabilities in a variety of clinical settings. Combined with high-speed helical imaging and a wider bore, this system offers both clinical and productivity advantages for hospital emergency and trauma departments.


 March 03, 2026
March 03, 2026 









