August 22, 2014 — VuCOMP Inc. has received market clearance for both M-Vu CAD and M-Vu Breast Density in Turkey.
The clearance marks another milestone as VuCOMP continues to expand the availability of its products internationally. “We are pleased to gain market clearance in Turkey and continue our focus on increasing access to our advanced technologies globally,” said James Pike, president and chief technology officer, VuCOMP.
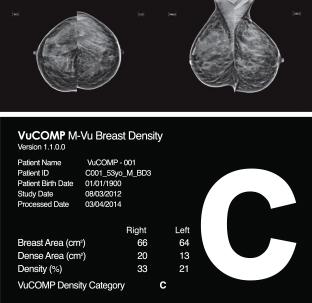
To date, VuCOMP has developed and commercialized two breast-imaging products, M-Vu CAD and M-Vu Breast Density. M-Vu CAD analyzes mammograms to mark areas consistent with breast cancer using sophisticated mathematical algorithms. M-Vu Breast Density is designed to advance the science of breast density measurement by identifying dense tissue in the breast that could be masking cancer.
Both products have been well received by leading radiologists and are used by clinics around the world. The company has additional cancer detection products currently under development.
For more information: www.vucomp.com


 February 23, 2026
February 23, 2026 









