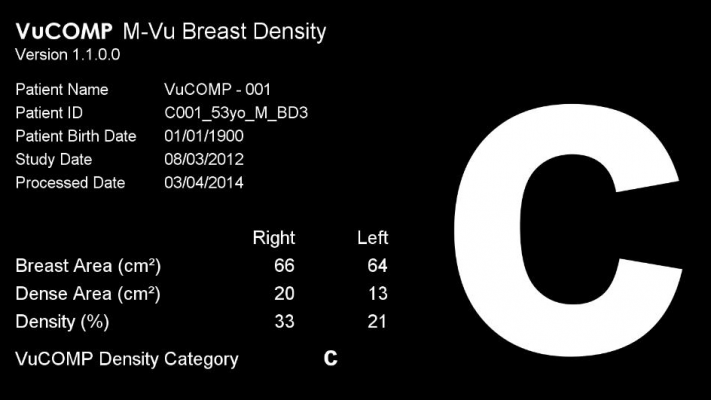
March 10, 2014 — The need to assess breast density as part of routine mammography is well documented and is required by legislation in many states. Vucomp’s U.S. Food and Drug Administration (FDA)-cleared M-Vu Breast Density automatically assesses breast density, providing consistent and accurate measurements calibrated to a panel of 13 expert radiologists.
M-Vu Breast Density evaluates mammograms in much the same manner as experienced radiologists do: by analyzing the structure and texture of the tissue, rather than simply estimating total fibroglandular volume. This exclusive approach using appearance-based analysis provides useful adjunctive information. The resulting M-Vu Breast Density category is analogous to the BI-RADS density category, which reflects the degree to which the mammographic image exhibits a breast pattern that could camouflage cancer.
For physicians, this means fast and consistent assessments. For patients, it means peace of mind.
"We find M-Vu Breast Density very beneficial in providing consistent accurate breast density reads across our practice,” said Christine A. J. Granfield, M.D. Medical Director for Breast Imaging, Baptist Health. “While we already were providing the breast density estimate in the dictated report, it is reassuring to know we are now reporting this information accurately to the primary physician, which in turn can effect their patient management."
M-Vu Breast Density is compatible with mammography systems of Carestream, Fujifilm, GE, Giotto, Hologic, Konica Minolta, Philips, Planmed and Siemens. The tower or rack server communicates over Ethernet network. The product can be combined with the M-Vu CAD system for a comprehensive set of tools to help radiologists find breast cancer earlier, and Vucomp resources are ready and available to ensure an efficient installation.
For more information: www.vucomp.com


 February 18, 2026
February 18, 2026 









