December 30, 2020 — Volpara Health announced two new research studies using AI-powered software to score breast density objectively and consistently to evaluate its impact in mammography and breast cancer risk assessment.
Volpara Density software uses a combination of X-ray physics and machine learning to generate an accurate volumetric measure of breast composition and remains the density measurement tool of choice for breast cancer research. With the two studies, which were presented at the virtual 106th Annual Radiological Society of North America (RSNA) meeting, Volpara has reached another research milestone with more than 300 journal articles and conference proceedings, including more than 150 peer-reviewed papers.
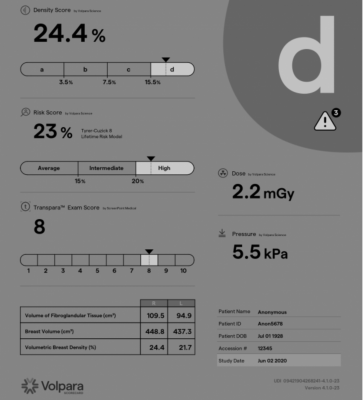
In the study, "A Tyrer-Cuzick Lifetime Risk Study to Determine the Impact of Breast Density on Risk Stratification," lead investigator Stamatia V. Destounis, M.D., Elizabeth Wende Breast Care and colleagues evaluated the risk calculations produced by Tyrer-Cuzick version 7 (TC7) and an updated Tyrer-Cuzick version 8 (TC8) model that can now include different breast density inputs. The results showed that TC7 identified 8.3% of women to be at high lifetime risk and TC8, with visual BI-RADS as the density input, identified 6.9% of women as high-risk. When Volpara's volumetric breast density percentage (VBD%) was used, 11.4% of women were determined to be high-risk. The use of VBD% in TC8 identified a greater number of high-risk patients and led to a change in medical management for 6.2% of the study population compared to TC7.
In another presentation, "Does Contrast Agent Change Breast Density Measurement in Low-Energy Contrast Enhanced Spectral Mammography?" Gisella Gennaro, Ph.D. and colleagues compared VBD% on both low-energy contrast-enhanced spectral mammography and conventional digital mammography images.
Results revealed no significant difference in VBD%, indicating that the presence of contrast agent doesn't impact Volpara's AI-powered breast density assessment in low-energy contrast-enhanced spectral mammography compared to conventional mammography.
"Volpara partners with radiologists to give women the most accurate information possible regarding their breast health. We believe that the best way to achieve this goal is through cutting-edge science and we are proud to collaborate with key researchers and clinicians around the globe to continuously improve women's health," said Ralph Highnam, Ph.D., founder and CEO of Volpara Health Technologies. "These latest studies continue to demonstrate seamless clinical integration of Volpara's AI-powered software to support objective breast density assessment and risk stratification."
Volpara's AI-technology has assessed the breast composition of more than 13 million women across 39 countries and is installed in more than 2,000 leading facilities worldwide, including top cancer centers in the United States.
For more information: www.volparahealth.com


 February 06, 2026
February 06, 2026 









