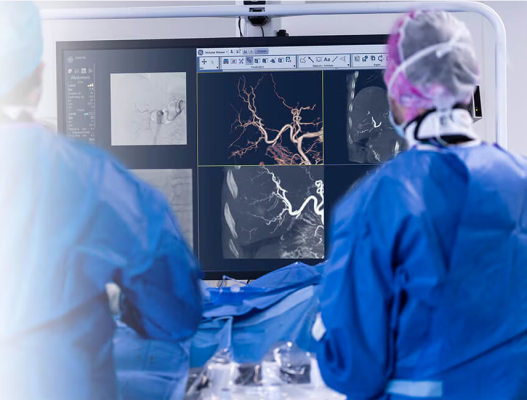
May 15, 2025 — GE HealthCare has launched CleaRecon DL, technology powered by a deep-learning algorithm, to improve the quality of cone-beam computed tomography (CBCT) images. This artificial intelligence (AI)-driven solution is designed to remove streak artifacts caused by the pulsatile nature of blood flow in the arteries and changes in the distribution of contrast during CBCT acquisitions in liver, prostate, neuro, and endovascular aortic repair procedures. CleaRecon DL recently received U.S. FDA 510(k) clearance and CE mark and will be available for use on the Allia platform.1
CBCT is used in interventional suites to provide cross-sectional imaging during procedures. However, the quality of CBCT reconstructed images may be diminished due to artifacts resulting from vessels’ pulsatility, which can reduce image clarity and accuracy. These limitations can impact the confidence in CBCT image interpretation and its adoption in routine clinical practice.2 Despite these challenges, CBCT remains crucial in interventional procedures for its ability to provide comprehensive visualization of anatomical structures and may enhance procedural accuracy.
“The introduction of CleaRecon DL represents a leap forward in the interventional suite and for the advancement of CBCT. By improving image quality and reducing artifacts, this technology can empower clinicians to perform procedures with greater precision and confidence,” said Arnaud Marie, General Manager, Interventional Solutions at GE HealthCare. “This solution builds on our portfolio of tools aimed at improving the user experience and workflow efficiency, enabling clinicians to deliver more accurate and effective interventions for enhanced patient outcomes.”
Deep learning is an AI technology that has become the state-of-the-art machine learning technique for image processing and is trained to output data and perform specific tasks.3 It is based on population representative data collection and thorough tests with clinical domain experts. CleaRecon DL harnesses deep-learning algorithms designed to provide clearer and more accurate imaging, enabling healthcare professionals to make better-informed decisions and improve their patient care. During clinical validation testing, a recent survey noted that in 98% of cases, CBCT images reconstructed with CleaRecon DL are clearer than conventional CBCT images. This technology was also shown to improve CBCT image interpretation confidence in 94% of cases.4
"CleaRecon DL takes CBCT to the next level, enabling clinicians to confidently use CBCT on patients with tools that help us provide the highest quality imaging and treatment across a wide range of clinical scenarios,” said. Dr. Charles Nutting5, Interventional Radiologist, Image Guided Therapy in Denver, Colorado. “This advancement improves our ability to perform precise interventions, with less manipulation of the image and eliminates artifacts that have historically hindered image clarity, ultimately helping improve the care clinicians can provide to patients.”
CleaRecon DL is available in the United States and European Union.6
For more information, please visit: https://www.gehealthcare.com/products/image-guiding-solutions/cone-beam-computed-tomography.
1 CleaRecon DL is an option in 3DXR designed to be used with Allia IGS 5 and Allia IGS 7 systems and requires AW workstation with Volume Viewer.
2 Matthias Barral, Olivier Chevallier, Francois H. Cornelis, Perspectives of Cone-beam Computed Tomography in Interventional Radiology: Techniques for Planning, Guidance, and Monitoring, Techniques in Vascular and Interventional Radiology, Volume 26, Issue 3, 2023, https://doi.org/10.1016/j.tvir.2023.100912.
3 Dede, A., Nunoo-Mensah, H., Tchao, E. T., Agbemenu, A. S., Adjei, P. E., Acheampong, F. A., & Kponyo, J. J. (2025). Deep learning for efficient high-resolution image processing: A systematic review. Intelligent Systems with Applications, 26, 200505. https://doi.org/10.1016/j.iswa.2025.200505
4 GE HealthCare data on file.
5 Dr. Nutting is a paid consultant for GEHC and was compensated for participation in this testimonial. The statements by Dr. Nutting described here are based on his own opinions and on results that were achieved in his unique setting. Since there is no “typical” hospital and many variables exist, i.e. hospital size, case mix, etc. there can be no guarantee that other customers will achieve the same results.
6 CleaRecon DL may not be available in all countries. Contact your GEHC sales representative for more information.


 February 13, 2026
February 13, 2026 









