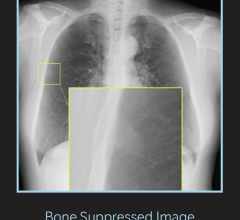
November 22, 2015 — Visaris Americas announced it has received 510(k) U.S. Food and Drug Administration (FDA) clearance on its digital radiography (DR) software, flat-panel detectors and various hardware offerings. The company will launch the full portfolio at the 2015 Radiological Society of North America (RSNA) annual meeting, Nov. 29-Dec. 4 in Chicago.
Avanse acquisition software is a central technology to the Visaris360 software portfolio. It is designed to integrate with the latest generation flat-panel detectors, enabling facilities to smoothly transition to a single software platform; reducing training and overall support costs; and providing complete integration and increased productivity of the entire X-ray department.
As part of the comprehensive Vision Family of fully-integrated DR systems, the Avanse platforms offers many features and provides full interoperability, improving the quality and reproducibility of clinical images while further reducing examination time.
For more information: www.visarisamericas.com

 February 10, 2026
February 10, 2026