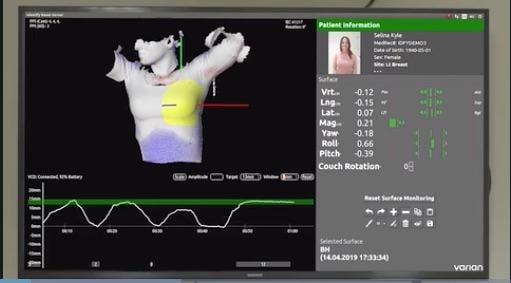
August 2, 2019 — Varian showcased systems and software from its cancer care portfolio, including the Identify Guidance System, at the recent American Association of Physicists in Medicine (AAPM) 2019 annual meeting, July 14-18 in San Antonio, Texas.
Identify is an automated patient identification, positioning and motion management system for radiation therapy. It incorporates patient safety, quality, efficiency and surface-guidance features in an automated workflow solution for surface-guided radiation therapy (SGRT).
Other Varian solutions showcased bring together artificial intelligence (AI), data analytics, science and technology, and include:
-
RapidPlan leverages machine learning to help clinicians achieve greater treatment plan consistency, efficiency and quality;
-
Multi-Criteria Optimization (MCO) improves the efficiency and quality of treatment planning by helping clinicians explore clinical tradeoffs when they vary plan parameters;
-
Noona is an intuitive solution for patients to report on their symptoms and outcomes, enabling better symptom monitoring, proactive management, and patient triage; and
-
ProBeam 360° system, designed for next-generation proton therapy, offers high-level clinical capabilities, a 360-degree gantry and RapidScan technology, which simplifies the process of motion management by delivering each field in a single breath-hold
Also on display was the full spectrum of Varian software solutions for: brachytherapy treatment planning and guidance, practice management, radiosurgery delivery, machine quality assurance, imaging informatics, image-guided dosimetry for Y90 selective internal radiation therapy (SIRT), tumor board management and more.
For more information: www.varian.com


 February 04, 2026
February 04, 2026 









