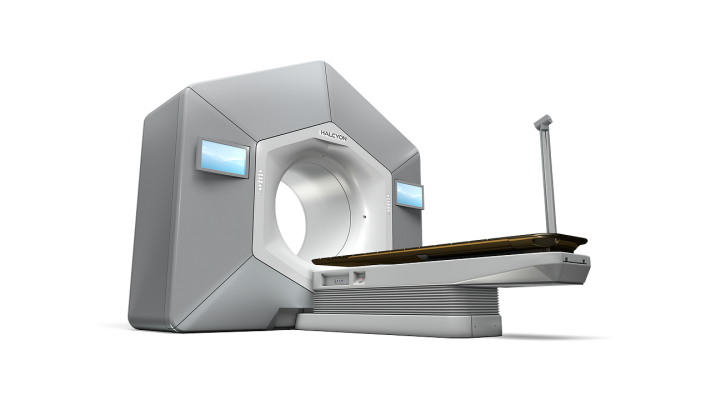
February 6, 2023 — Varian, a Siemens Healthineers company, announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA), as well as CE mark, for Halcyon and Ethos radiotherapy systems featuring Varian's HyperSight imaging solution. On February 1, a cancer patient at Penn Medicine became the first person in the world to be treated on a Halcyon system equipped with HyperSight.
HyperSight enables clinicians to capture high-quality images of patients during their daily radiation treatments. These images are used for daily localization of patient tumors, and HyperSight now enables them to be used for replanning and adaptation to patient and tumor changes. HyperSight's cone-beam computed tomography (CBCT) technology delivers larger images with better contrast, and is 10 times faster than conventional linear accelerator-based imaging systems, saving time for patients and creating the potential to significantly enhance the patient experience.
Traditional CBCT imaging can take up to 60 seconds and may require patients with tumors that move with breathing — including lung, liver, and left breast tumors — to hold their breath several times to acquire a full, clear image. HyperSight can acquire images in six seconds, potentially minimizing patient discomfort and anxiety and contributing to clearer images due to reduction of motion related image blurring.
The quantitative information about patient anatomy (Hounsfield Units) contained within HyperSight images allows, for the first time, for radiation dose distributions to be calculated directly on CBCT images. This calculation previously required a patient to make an additional trip to a separate CT scanner used for treatment simulation and planning. With HyperSight, this calculation can be performed directly on the linac-based CBCT image during the normal course of treatment. As a result, this technology may help adapt treatment to adjust to anatomical changes to the tumor and surrounding organs, which can change from day to day during treatment.
"Varian has long been focused on pioneering innovations that can enhance the radiotherapy experience and outcomes for both patients and cancer care teams. Becoming a Siemens Healthineers company has given us an opportunity to sharpen and accelerate these efforts, with a focus on connecting the power of imaging inside and outside the treatment room," said Kevin O'Reilly, President of Radiation Oncology Solutions at Varian. "HyperSight reflects the power of our expanded view of the patient journey and robust imaging technology. We look forward to working with our clinical partners and the broader radiotherapy community to drive adoption and further advancement of this technology, as we continue our work to transform the patient pathway and deliver on our mission to create a world without fear of cancer."
As a longtime collaborator and the first cancer center in the world to adopt Varian's Halcyon system, Penn Medicine has played a key role in supporting the advancement of radiation therapy innovations, including HyperSight.
"Bringing this technology to patients is another step forward in our work to help develop and assess cutting-edge treatment technologies that improve care for cancer patients," said James Metz, MD, chair of Radiation Oncology and leader of the Roberts Proton Therapy Center at Penn.
For more information: www.varian.com


 February 09, 2026
February 09, 2026 









