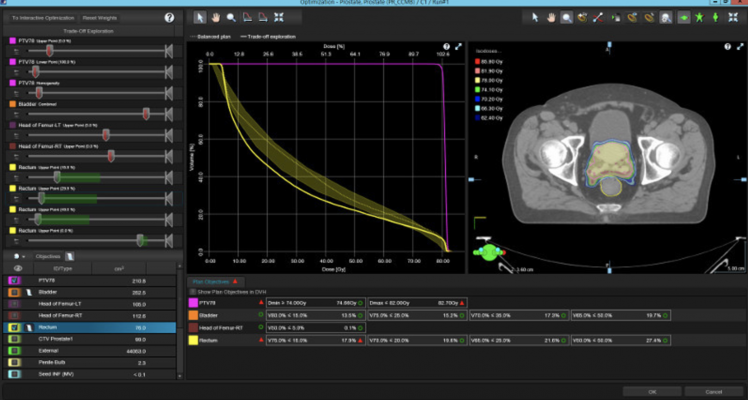
April 6, 2020 — Driven by its Intelligent Cancer Care approach in developing new solutions that use advanced technologies like machine learning, Varian announced the newest release of its treatment planning system, Eclipse v16. This new release includes intelligent features such as RapidPlan PT, the first clinical application of machine learning in proton treatment planning, and RT Peer Review, which is a collaborative workspace designed to streamline and accelerate the peer review process for radiotherapy treatment plans.
Previously only available for photon-based radiotherapy treatment planning, RapidPlan is knowledge-based treatment planning software that enables clinicians to leverage knowledge and data from similar prior treatment plans to quickly develop high-quality personalized plans for patients. This knowledge-based planning software is now available for proton treatment planning with RapidPlan PT. The software also allows dose prediction with machine learning models that can be used as a decision support tool to determine which patients would be appropriate for proton or photon therapy. Varian is the first vendor in the industry to offer machine learning capability in both proton and photon treatment planning.
"With the number of operational proton treatment rooms continuing to increase, there is a need for experienced proton therapy clinicians," said Kolleen Kennedy, chief growth officer, president, Proton Solutions, Varian. "RapidPlan PT helps bridge the learning curve, allowing established centers to share their models and clinical experience. The machine learning in RapidPlan PT has the potential to reduce proton treatment plan optimization from a one to eight hour process, as reported by clinical proton centers, to less than 10 minutes, while also potentially improving plan quality."
In many radiotherapy departments, radiation therapy peer review meetings have been routinely integrated into the clinical QA process for safer healthcare delivery for the patient. Although the relevant patient information is manually retrievable from the clinical database, there is currently no efficient and effective platform to support these peer reviews. The RT Peer Review feature in Eclipse v16 is designed for the oncology community to seamlessly integrate this review process into their normal clinical workflow by automatically presenting the necessary information that is required for peer review.
Eclipse v16 has received the CE mark and is 510(k) pending. For more information on Eclipse v16, visit www.varian.com/eclipse.


 February 04, 2026
February 04, 2026 









