December 10, 2013 — TomTec Imaging Systems GmbH announced the U.S. Food and Drug Administration (FDA) 510(k) clearance for its new TomTec-Arena software solution.
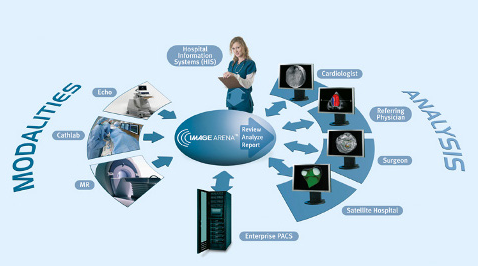
TomTec-Arena is a suite of clinical applications that review, analyze and quantify medical image data in multiple dimensions (2-D and 3-D/4-D) as well as from multiple modalities such as ultrasound, angiography, magnetic resonance (MR) and nuclear.
TomTec-Arena combines TomTec’s entire cardiology and radiology application portfolio in one product. It can analyze standard 2-D DICOM as well as 2-D and 3-D proprietary data formats from Philips, GE, Siemens, Toshiba and others.
“Time efficient image review, measurements and analysis plays a very important role to improve the clinical workflow,” said Bernhard Mumm, president and chief medical officer, TomTec. “TomTec-Arena has been developed to be integrated into our PACS partner systems as well as into our Image-Arena platform.”
"This new product helps radiologists and cardiologists to optimize their workflow towards clinical outcome and efficiency,” said Gregor Malischnig, manager of the healthcare IT business unit, TomTec. “It offers a wide range of routine and advanced measurements for various clinical questions.”