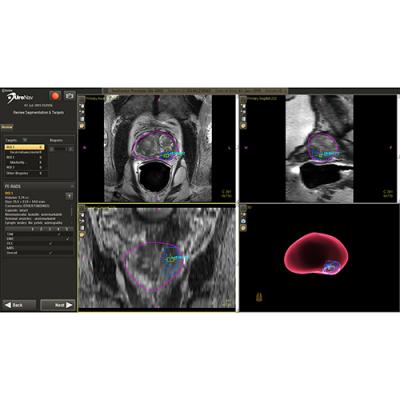
July 17, 2017 — Radiologists from Synergy Radiology Associates (SRA) in Houston are using the power of 3-D medical imaging and subspecialty radiology to provide better patient care for men with suspicious prostate cancer findings.
The American Cancer Society reports that prostate cancer is the most common cancer and second leading cause of death among U.S. men. Therefore, when positive digital rectal exams (DRE) and elevated PSA (prostate-specific antigen) levels raise suspicion of cancer, a sampling (biopsy) of prostate gland tissue is recommended. These tissue samples are used to help diagnose and determine the grade, or Gleason score, of prostate cancer.
Unfortunately, current biopsy methods are not always efficient at finding actual cancer, leading to repeat and sometimes unnecessary biopsies. That’s where magnetic resonance imaging (MRI) and the UroNav Fusion Biopsy System come in.
“The UroNav approach tends to find those lesions that are more likely to become cancer and require treatment,” said SRA radiologist Larry Schock, M.D., specializing in body imaging and diagnostic radiology. “Radiologists appreciate the computer-aided 3-D imaging capabilities supporting UroNav that improve diagnosis, detection and treatment planning; our male patients appreciate a quicker, more certain diagnosis with fewer repeat or unnecessary biopsies. It’s also an excellent tool for diagnosing high-risk or aggressive prostate cancers.”
If a suspicious lesion is identified using prostate MRI, the UroNav DynaCAD workstation allows SRA radiologists to quickly view and work with 3-D images of the prostate for highly accurate diagnosis and location of suspicious tissue. Urologists then use live ultrasound plus existing 3-D MRI images during biopsy to obtain the best tissue samples.
Before UroNav, physicians relied primarily upon transrectal ultrasound (TRUS) to visualize the prostate gland and sample multiple areas of the prostate in an effort to detect possible cancer cells. However, with TRUS biopsies, cancer can often go undetected, according to recent published research.
“With UroNav, radiologists and urologists work in tandem, using the power of MRI, radiology subspecialty expertise and ultrasound guidance to accurately detect cancer 90 percent of the time,” said Schock. “This combined technology offers a truly new biopsy option for patients with suspicious prostate findings or who are under active surveillance.”
UroNav incorporates prostate image information from pre-biopsy MRI, computer-aided image enhancement and ultrasound-guided needle biopsy. This “fusion” of MRI and ultrasound results in targeted biopsies that are much better at detecting true cancer while avoiding the detection of low-risk or slow-growing cancer.
“Prostate is one of the most common but most curable cancers in men, and early, accurate detection can make all the difference in surviving,” said SRA President Walid Adham, M.D. “It’s incredibly rewarding for our radiology team to work with this advanced UroNav system to improve diagnosis, decrease worry and provide the best care possible to our patients.”
The UroNav fusion biopsy is an outpatient procedure that takes about 15 to 20 minutes and can be performed very shortly after the diagnostic MRI.
UroNav is recommended for patients with a PI-RADS (Prostate Imaging-Reporting and Data System) score of 3 or higher, negative prior TRUS biopsies with a continued elevated or rising PSA, or positive digital rectal examination with a negative TRUS biopsy.
For more information: www.invivocorp.com


 February 13, 2026
February 13, 2026 









