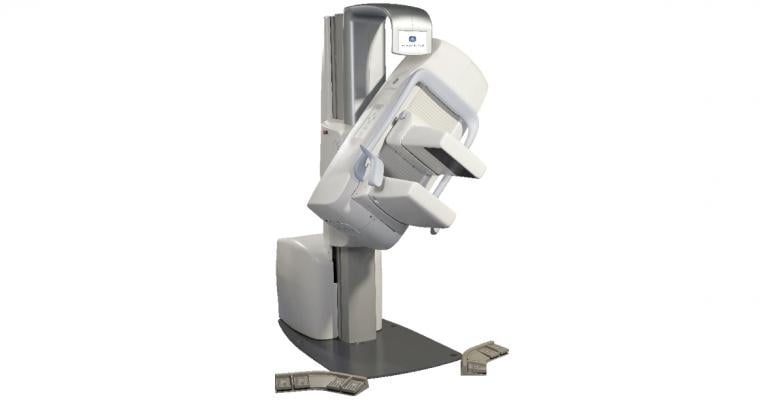
October 23, 2013 — St. Luke’s Medical Center is the first hospital in the Texas Medical Center to offer a molecular breast
imaging (MBI) device that is sensitive enough to detect tiny tumors. GE Healthcare’s Discovery NM 750b is designed to help improve detection of breast cancer, even in women with dense breasts where results of
mammography may be inconclusive.
“The Discovery system is an important advancement in the early detection and diagnosis of breast cancer, but for women with dense breasts, it could truly be a lifesaver,” said Eric Ladwig, operations manager, nuclear medicine, St. Luke’s Medical Center. “Women with dense breasts have an increased risk of breast cancer and this condition can impact the quality of a traditional mammogram. MBI is not affected by breast density, resulting in a clear image and great confidence that the patient is cancer free or truly needs to have a biopsy.”
MBI is a comfortable examination, as only light immobilization pressure is required during studies versus a traditional mammography.
During an MBI procedure with Discovery, a patient receives a tracer injection that locates metabolically active tumors by blood flow. Because the tumor metabolizes the isotope to a higher degree than normal tissue, it will appear as a “hot spot” on the scan.