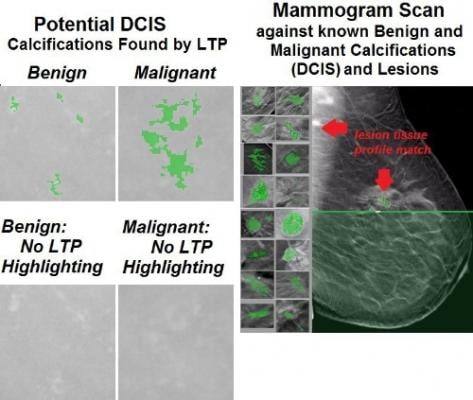
A California software company, www.YourScan.org, has put out a call to women who have had to face the difficult diagnosis of breast cancer: Donate your mammogram images to a crowdsourcing campaign and help confirm that a new visual recognition software presents a breakthrough in early detection and distinguishing benign from malignant precursors (DCIS) and lesions through the use of "Lesion Tissue Profiling (LTP)."
In a pilot test conducted over the last three years, the company has shown that its Lesion Tissue Profiling technology is capable of accurately detecting the shapes of malignant DCIS and tumors (lesions) otherwise not visible to the human eye. The study further indicated that these lesions have a limited number of basic shapes that can be rendered as distinct tissue profiles common to all malignancy. The implication is that these profiles may be used as a tool in routine screening of all mammography. This could present an important breakthrough — the first automated digital search of the more than 30 million mammograms done in the US each year, a search that would offer valuable diagnostic insight.
The company, YourScan.org, has built a proprietary database specifically designed to store mammographic images in a format allowing for rapid and precise comparison of billions of tissue profiles. The data is highlighted using a visual-based color illustrator and the technology catalogues each shape – assembling a "tissue profile" that can be matched with other tissue profiles. Searching "healthy" mammograms using those tissue profiles has revealed that the technology can detect "precursor" (DCIS) tissue profiles in digital mammograms, both two-dimensional and newer 3-D images produced by tomosynthesis technology. The Company is asking women diagnosed with breast cancer to provide their entire series of mammograms to compare earlier mammograms to the later one that resulted in a definitive diagnosis; identifying and cataloging precursor and lesion tissue profiles found in their mammograms. These newly identified tissue profiles will be added to the tissue profile library, and used to search other mammograms for breast cancer precursors and lesions.
For more information: www.YourScan.org.


 February 18, 2026
February 18, 2026 









