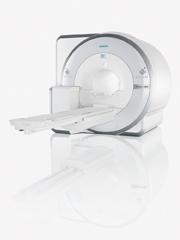
July 13, 2011 – Based on its recent analysis of the medical imaging market, Siemens Healthcare was given the 2011 North American Frost & Sullivan Award for New Product Innovation. Siemens' Biograph mMR is the first scanner capable of simultaneously acquiring whole-body positron emission tomography (PET) and magnetic resonance imaging (MRI) data. "While the ability to fuse and spatially register PET and MRI data has been available for several years, no manufacturer has successfully integrated these two modalities into a single-gantry, whole-body scanner," said Frost & Sullivan research analyst Roberto Aranibar. Simultaneous acquisition of PET and MRI data reduces overall procedure time by providing PET/MRI in a single scan, thus eliminating any time required for patient transport. Faster procedure times improve the patient experience and enables faster delivery of images to clinicians for review. The collection of MRI attenuation data during diagnostic scanning also reduces procedure time and improves clinical workflow. With a footprint comparable to a standard, high-field MRI scanner, the Biograph mMR can be sited in existing MRI rooms, eliminating renovation costs for customers seeking to replace an existing MRI. "The ability to be used in an MR-only capacity, when PET is not clinically indicated, helps keep utilization of the scanner high, and the ability to perform PET/MRI within the time frame of a typical MRI procedure helps maximize patient throughput and maintain good clinical work-flow," said Aranibar. Based on its imaging characteristics and capabilities, PET/MRI is initially expected to find the strongest interest among clinicians and researchers in neurology, oncology, cardiology and pediatric imaging.* Each year, Frost & Sullivan presents this award to the company that has demonstrated excellence in the following categories: innovative element of the product, leverage of leading edge technologies, value-added features/benefits, increased customer ROI and customer acquisition/penetration potential. Frost & Sullivan Best Practices Awards recognize companies in a variety of regional and global markets for demonstrating outstanding achievement and superior performance in areas such as leadership, technological innovation, customer service and strategic product development. Industry analysts compare market participants and measure performance through in-depth interviews, analysis and extensive secondary research in order to identify best practices in the industry. *MR scanning has not been established as safe for imaging fetuses and infants under two years of age. The responsible physician has to decide about the benefit of the MRI examination in comparison to other imaging procedures. For more information: www.awards.frost.com, www.siemens.com/healthcare

 March 05, 2026
March 05, 2026