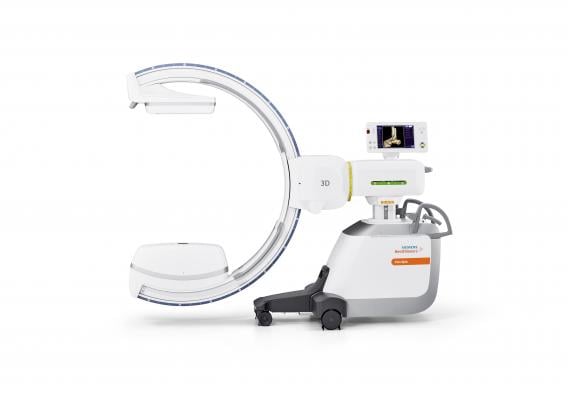
November 6, 2018 — Siemens Healthineers announced the U.S. Food and Drug Administration (FDA) clearance of the Cios Spin, a new mobile C-arm that delivers precise 3-D images for intraoperative quality assurance.
Capable of integrating seamlessly into the clinical routine, the Cios Spin provides 3-D computed tomography (CT)-like imaging for orthopedic, trauma and spine surgery. These 3-D images can help reduce the rate of revisions with intraoperative evaluation as well as the need for post-operative CT. Cios Spin provides versatility to support both 2-D and 3-D imaging for a wide variety of procedures, including vascular imaging. The system’s NaviLink 3-D digital navigation provides easy-to-use connectivity to surgical navigation.
Cios Spin is equipped with state-of-the-art flat panel detector technology and is available with a range of optional software packages. The Easy 3-D package ensures fast, efficient setup and image acquisition. The Screw Scout package enables system software to recognize and automatically label screws in a 3-D X-ray image, saving time and effort for the surgeon.
The system’s high generator power addresses the challenge of imaging large patients and dense anatomy to enable precise clinical evaluation of images. And the Cios Spin is the first commercially available mobile C-arm with an antimicrobial coating for comprehensive infection control.
For more information: www.usa.healthcare.siemens.com


 January 22, 2026
January 22, 2026