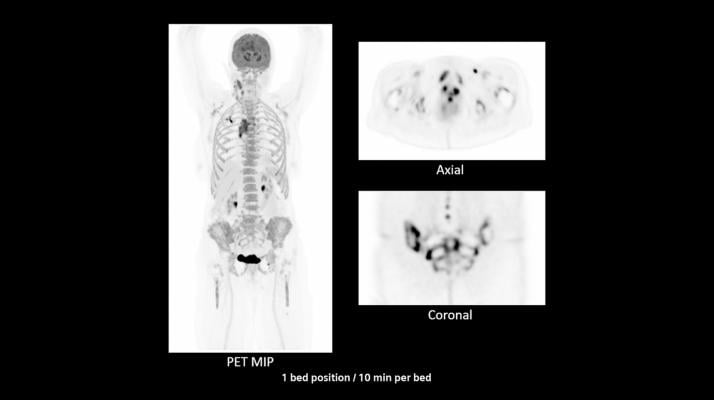
March 8, 2021 — Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the Biograph Vision Quadra, a positron emission tomography/computed tomography (PET/CT) scanner designed for clinical use as well as translational research – or the application of scientific research to create therapies and procedures that improve health outcomes. In this manner, the Biograph Vision Quadra expands precision medicine.
In addition to the 3.2 mm silicon photomultiplier (SiPM) detector technology and Time of Flight (ToF) performance that are cornerstones of the established Biograph Vision PET/CT scanner, the Biograph Vision Quadra has an extended 106 cm axial field of view (FoV), which is four times the PET axial FoV of the Biograph Vision 600. These technological features deliver significantly increased effective sensitivity¹ and allow the clinician to image the average patient dynamically from the top of the head to the thigh in just one position. Thanks to the scanner’s extended axial FoV, the clinician can examine patient anatomy during radiopharmaceutical uptake over time. The combination of SiPM detectors and extended axial FoV permits more anatomical coverage in one bed position than a standard PET/CT scanner, enabling fast scanning at low patient radiation dose.
“The Biograph Vision Quadra breaks through current clinical scanner limitations by simultaneously imaging all vital organs in a single field of view,” said John Khoury, Head of the Molecular Imaging business at Siemens Healthineers North America. “This new system helps open the door to better understand disease.”
Since the Biograph Vision Quadra can be sited in the same clinical space as traditional PET/CT scanners, institutions do not need to build a large new room to house the scanner.
For more information: siemens-healthineers.us/quadra


 March 06, 2026
March 06, 2026 









