October 17, 2011– GE Healthcare announced that the U.S. Food and Drug Administration (FDA) gave 510(k) clearance of an innovative technology to aid the physician in breast cancer diagnosis. GE estimates that by 2020, more than 1 million women worldwide will be examined using SenoBright, and this technology can help lead to more productive diagnosis paths for nearly 250,000 women.
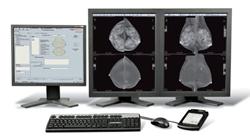
GE Healthcare’s new SenoBright contrast-enhanced spectral mammography (CESM) technology is designed to produce contrast-enhanced images of the breast using a legally approved X-ray contrast agent and a dual energy acquisition technique. Launched in 2010, SenoBright is already in use at 17 major mammography centers in France, Spain, Italy, Belgium, Germany, Austria and Japan.
SenoBright uses X-rays at multiple energies to create two separate but almost simultaneous exposures. The resulting images specifically illuminate and highlight areas of iodinated contrast.
“CESM presents two images per mammographic view, one that looks like standard mammography and a second image that showed the contrast-enhanced areas that can help localize a lesion. As the images are familiar, it can therefore be easily reviewed by surgeons and oncologists. Moreover, in terms of workflow, a CESM exam takes from 5 to 10 minutes," said Clarisse Dromain, M.D. Gustave Roussy Cancer Institute, France.
The diagnostic challenge
“Worldwide, more than 1.2 million people annually are diagnosed with breast cancer. Since 1965, GE Healthcare has made significant progress in providing solutions for breast cancer detection and diagnosis that really bring a change to people’s lives. Today through ‘healthymagination,’ we continuously develop innovations to reduce costs, increase access and improve quality and efficiency of healthcare delivery around the globe,” said Anne LeGrand, GE Healthcare’s vice president and general manager of the company’s global X-ray business.
Clarity of results
Digital mammography is considered a relevant means of breast cancer screening, delivering proven clinical outcomes. The sensitivity and specificity of images can, however, be affected by a range of factors. Dense breast tissue can overlap with lesions, which are not always visible on an X-ray, and radiologists’ interpretation of images can vary. Inconclusive digital mammography presents a range of challenges to healthcare professionals and patients. Ambiguity can result in diagnostic error.
Same staff, same equipment— same day
SenoBright is intended to allow for a procedure to be conducted by the same staff, using the same mammography equipment, potentially on the same day as a traditional screening exam, thereby helping medical professionals to cut the critical time patients often have to wait from detection to diagnosis. GE Healthcare estimates that 2,500 digital mammography systems upgradeable to SenoBright are in clinical use today – delivering an excellent investment for these customers-- and providing added functionality to an existing and vital tool.
CESM technology is intended to work as an upgrade to GE Healthcare’s Senographe DS and Senographe Essential digital mammography equipment. GE’s Senographe platforms are full-field digital mammography systems designed to meet a wide range of clinical needs, from screening to diagnostic and interventional procedures.
For more information: www.gehealthcare.com


 February 17, 2026
February 17, 2026 









