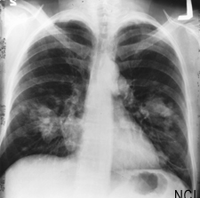
August 11, 2011 -- The New England Journal of Medicine's recent publication of the landmark National Lung Screening Trial (NLST), along with two congressional initiatives and an industry report, herald a much brighter future for lung cancer patients.
Forty-three million Americans suffer from a chronic pulmonary disease, such as lung cancer, chronic obstructive pulmonary disease (COPD), asthma and cystic fibrosis. Pulmonary diseases account for three out of the top five disease states with the highest incidence of mortality. Lung cancer is the deadliest of all cancers, causing nearly 30 percent of all cancer-related deaths, killing over 200,000 Americans annually. In 2009, the Lung Cancer Alliance confirmed that 70,490 women perished from lung cancer, more than all other female-related cancers (breast, cervical, uterine, and ovarian) combined.
Larry Gerrans, CEO and co-founder of Sausalito, Calif.-based Sanovas, a manufacturer of therapy-enabling technologies for the treatment of pulmonary disease and lung cancer, said, "As we have seen with other chronic diseases, early detection has proved to save lives and relieve the emotional and financial hardship these diseases have on families and on the health care system. The NLST promises new hope for patients who otherwise would not know that lung cancer has taken hold of them."
Published August 4, the reduced lung-cancer mortality with low-dose computed tomographic (CT) screening study found a 24.2 percent increase in the detection of lung cancer using low-dose CT over chest X-ray alone. The study, which enrolled 53,454 persons at high risk for lung cancer at 33 United States medical centers, also found a 20 percent reduction in mortality from lung cancer in patients who were screened with a low-dose CT.
"This is the first time that we have seen clear evidence of a significant reduction in lung cancer mortality with a screening test in a randomized controlled trial. The fact that low-dose helical CT provides a decided benefit is a result that will have implications for the screening and management of lung cancer for many years to come," says Christine Berg, M.D., the National Cancer Institute project officer for the study.
Congress, in its effort to reduce the cost burden of lung cancer on the gross domestic product (GDP) of the country, has architected two legislative initiatives, in response to the national call to action on lung cancer. The bi-partisan, bi-cameral Lung Cancer Mortality Reduction Act, originally sponsored by Diane Feinstein (D-Calif.), was recently reintroduced in the 112th Congress. The Senate Bill (S.752) and its House counterpart (HR 1394) would establish the first-ever multi-agency comprehensive program targeted at lung cancer.
The "Medicare Lung Cancer Early Detection Promotion Act of 2011" seeks to amend title XVIII of the Social Security Act to provide reimbursement for chest radiography services that use computer-aided detection (CAD) technology for the purpose of
early detection of lung cancer.
Markets and Markets (M&M), a global market research and consulting company based in the United States, declared in its July 2011 analysis that "the market for lung cancer surgeries alone was valued at $36 Billion in 2010 and expected to grow to $45 Billion by 2015."
For more information: www.sanovas.com


 February 09, 2026
February 09, 2026 









