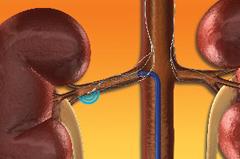
March 30, 2011 – Interventional radiologists have completed the first human randomized controlled trial of therapeutic renal denervation, or RDN. RDN is a procedure that uses a catheter-based probe inserted into the renal artery that emits high-frequency energy to deactivate the nerves near the kidneys (or in the renal artery) that are linked to high blood pressure. The researchers say these results confirm that RDN may be an effective therapy for reducing—and consistently controlling—resistant hypertension when current medications have failed. The results were presented at the Society of Interventional Radiology's 36th Annual Scientific Meeting in Chicago. "Renal denervation, a minimally invasive, effective treatment, appears to be safe in the short term with a low incidence of local complications. Its efficacy to lower blood pressure in patients with resistant high blood pressure will be better evaluated with the results of a subsequent trial," said Marc R. Sapoval, M.D., Ph.D., professor of clinical radiology and chair of the cardiovascular radiology department at Hopital Europeen Georges-Pompidou in Paris. "After six months, 39 percent of patients receiving the endovascular denervation treatment had reached the recommended blood pressure level and, overall, 50 percent of patients showed a measurable benefit of the intervention." The renal sympathetic system, the small nerves that carry signals to and from the brain and kidney, plays an important role in regulating blood pressure. However, the disruption of these nerve fibers has a positive effect on blood pressure levels. "Given its impact on the central sympathetic drive, endovascular renal denervation may have applicability in additional disease states such as heart failure, cardio-renal syndrome, hepato-renal syndrome, and in the prevention of progression of chronic kidney disease and hypertension in end-stage renal disease—with the added benefit of helping to raise public awareness on the dramatic burden of this disease," said Sapoval. This study targeted only patients with resistant essential hypertension, which means that a doctor couldn't find any reason for the condition. Sapoval said the causes of high blood pressure can be wide-ranging, such as a benign tumor in the adrenal gland, stenosis of the renal artery, the taking of certain prescription drugs or other factors. By randomized assignment, 106 adult patients with uncontrolled hypertension received either oral medication or the renal denervation treatment. Six months after the intervention, systolic pressure fell an average of 32 mmHg and diastolic pressure fell an average of 12 mmHg. This initial cohort has been expanded to a multicenter randomized controlled trial at 24 international sites. Sapoval conceded that this was a small study, that the work still experimental, and that renal denervation should be performed only by interventional radiologists on screened patients in strictly controlled academic and/or research settings. However, he noted that it shows great promise for those suffering from resistant hypertension. Sapoval said that the patients had a short hospital stay for safety reasons, but that the treatment might possibly be performed in an outpatient clinic in the future. For more information: www.SIRweb.org


 July 25, 2024
July 25, 2024 









