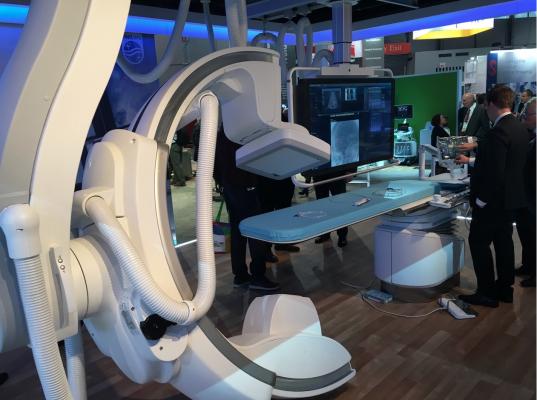
January 17, 2019 — Philips announced the launch of Azurion with FlexArm, designed to enhance positioning flexibility for image-guided procedures.
During increasingly complex interventions, clinicians need to quickly and easily visualize critical anatomy and identify changes to the patient during the procedure. Azurion with FlexArm includes a set of innovations that makes it easier for the clinician to perform imaging across the whole patient in both 2-D and 3-D. As the clinician moves the system, the image beam automatically maintains alignment with the patient, allowing more consistent visualization and enabling them to keep their focus on the treatment.
“With FlexArm, Philips’ engineers have overcome near-impossible geometric and mechanical barriers to enable clinicians to achieve clinical excellence in image-guided therapy,” said Barry T. Katzen, M.D., founder and chief medical executive of the Miami Cardiac & Vascular Institute, Baptist Health South Florida. “FlexArm enables us to dramatically optimize procedures around the patient: We can get the optimal view of what’s going on inside the patient without encumbering all of the clinicians that are working around the table. The result is an innovation that’s not only clinically important but also very simple and intuitive to use – a critical factor in the heat of a complex procedure.”
The range and complexity of diseases that can be treated with minimally invasive procedures continues to expand. Correspondingly, the procedures themselves are also becoming more complex, requiring more physicians from different disciplines to be at the patient’s tableside, working together in a highly coordinated way. As a result, the clinical team is required to carry out increasingly challenging procedures in a highly constrained environment.
Azurion with FlexArm’s design provides exceptional flexibility and intuitive control, according to Philips. Powered by a smart kinematic engine, the system moves on eight different axes, all controlled with its single ‘Axsys’ controller. Simulation tests with clinicians have demonstrated the system’s potential to significantly reduce the repositioning of the patient, staff and equipment to improve access for minimally invasive procedures, including those that enter the body through the patient’s wrist (radial access), and to reduce the risk of unintentional pulling of wires and tubes, as well as significant time savings [1]. The system is ideally suited for hybrid operating rooms (ORs) that cater to multiple specialties in one room, such as a combination of surgical and endovascular procedures.
The Philips Azurion 7 C20 with FlexArm is CE marked and has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
For more information: www.philips.com/flexarm
Reference
[1] Uselab simulation test with 15 clinicians.


 January 22, 2026
January 22, 2026