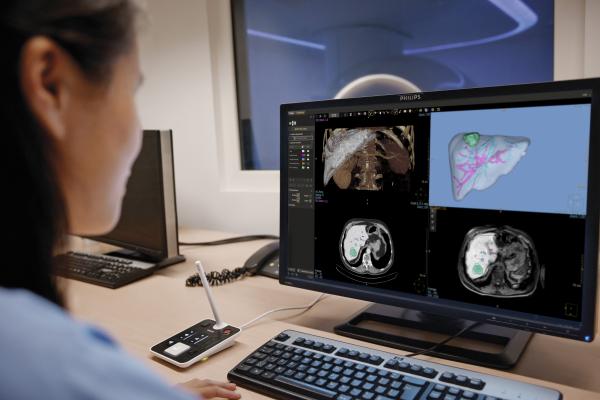
February 27, 2019 — Philips announced the launch of IntelliSpace Portal 11, the latest release of the company’s comprehensive, advanced visualization and quantification software. Launching at the 2019 European Congress of Radiology (ECR), Feb. 27-March 3 in Vienna, Austria, the new version further extends the clinical innovation of the portal, with enhancements to improve workflow efficiencies, bridging secure data sharing between systems within the hospital network to address the security and privacy needs of customers globally.
Philips IntelliSpace Portal is an advanced visualization and analysis software solution designed to support diagnostic process, follow-up and communication across clinical domains and modalities, through a connected and secure workflow. The solution is a multimodality and multivendor comprehensive suite of advanced visualization solutions for radiology. It can scale to fit large scale enterprises, helping to maximize resources by leveraging analytical tools.
Philips IntelliSpace Portal 11 offers a seamless integrated portfolio of clinically rich applications for neurology, oncology and cardiology domains. The new version is focused on enhancing clinical workflow and automation via adaptive intelligence (aka artificial intelligence, or AI) functionalities, combining clinical data from multimodalities across the health continuum. The latest iteration also includes a new integrated Nuclear Medicine Viewer by Mirada Medical designed to support clinical challenges and productivity, with a dedicated optimized workflow for quantitative patient follow-up.
IntelliSpace Portal 11 introduces new zero-footprint viewer capabilities running directly through a web browser to provide anywhere access to advanced visualization data review during multi-disciplinary meetings inside or outside the hospital. Users can consult and share findings online with real-time peer to peer interaction. The viewer also offers scalability from a single department to an enterprise-wide solution. Philips noted that the zero-footprint viewer is not intended for diagnostic review.
Supporting patient data confidentiality, integrity and availability are part of the IntelliSpace Portal 11 security policy. The solution can be tailored to the customer environment, allowing flexible settings for patient and role-based access controls, designed to protect sensitive data from unauthorized access.
For more information: www.usa.philips.com/healthcare


 March 06, 2026
March 06, 2026 









