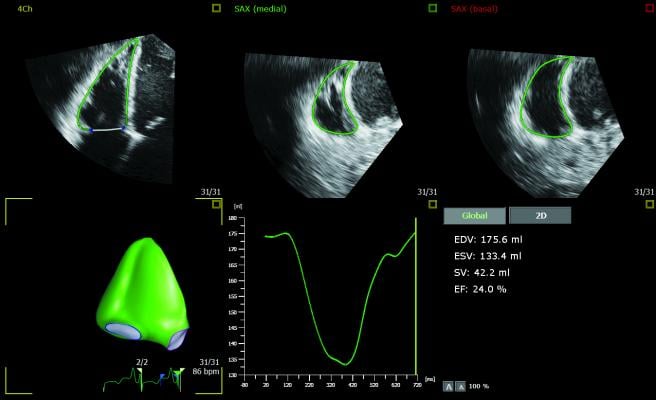
3D Auto RV application image courtesy of Philips Healthcare

AutoStrain LV application image courtesy of Philips Healthcare
July 2, 2019 — Philips recently announced new advanced automation capabilities on its Epiq CVx and Epiq CVxi cardiac ultrasound systems. With Release 5.0, both systems now include automated applications for 2-D assessment of the heart, as well as robust 3-D right ventricle volume and ejection fraction measurements, making accurate exams faster and easier to conduct.1 Together, the new applications provide clinicians with the means to confidently evaluate the heart’s function, increasing diagnostic confidence for patients with pulmonary hypertension, congenital heart disease, coronary disease and heart failure.
According to Philips, the company is leveraging artificial intelligence (AI) to make echo exams easier, faster and more reproducible, eliminating barriers to accessing high-quality care. The company said the new release of Epiq CVx reduces the number of touches of the system by 21 percent in each exam, which is equivalent to more than 400 exams each year.2
The AutoStrain LV application uses advanced Automatic View Recognition technology to identify the different views of the heart, providing high-quality visualization and analysis of left ventricular function – extremely important diagnostic information for patients at risk of developing cardiovascular disease. Also available are AutoStrain LA and AutoStrain RV, applications which automate the measurement of left atrial and right ventricular longitudinal strain, respectively. By creating reliable and reproducible strain measurements for the left ventricle, left atrium and right ventricle, the AutoStrain LV, LA and RV applications support clinicians treating patients with atrial fibrillation, arrhythmia and other complex heart conditions.
The 3D Auto RV application segments, identifies the borders of and aligns the views of the right ventricle, enabling clinicians to carry out the quantification and check the measurements in as little as 15 seconds.3
These new applications expand on the advanced automation applications already available on the Epiq CVx platform, including Dynamic HeartModel, which provides a clear vision of the heart’s chambers and how well they are pumping blood – specifically on the left side, where heart failure often begins. A recent study of Dynamic HeartModel published in the European Heart Journal – Cardiovascular Imaging concluded that ‘the automated algorithm can quickly measure dynamic left ventricle and left atrial volumes and accurately analyze ejection/filling parameters’.4
The new release also adds diagnostic capabilities to the Epiq CVxi. Tailored for use in the interventional lab, the system can now also be used in the echo lab.
Beyond monitoring overall heart function, new medical fields such as cardio-oncology are using echocardiography to better assess heart health during chemotherapy, which can damage the heart if the dosage is not closely monitored. Cardio-oncology has increased the global demand for echocardiography as clinicians need to perform a deep analysis on cardiac images throughout the patient’s treatment, ensuring measurements are consistent from one exam to the next so that the results can be reliably compared.
Philips unveiled Epiq CVx Release 5.0 alongside its full suite of solutions for diagnosis and interventional guidance during the annual meeting of the American Society of Echo (ASE), June 21-25 in Portland, Ore.
For more information: www.usa.philips.com/healthcare
References
1. The Philips CVx and CVxi 5.0 Diagnostic ultrasound systems are available for sale globally. The AutoStrain LV, LA, RV applications and the Dynamic Heart Model application are CE marked and U.S. Food and Drug Administration (FDA)-cleared and available for sale in Europe and in the U.S. The AI-enabled 3D AutoRV application, designed using a machine learning-based algorithm, is CE marked and FDA 510(k) pending, and is therefore available for sale in Europe but is not available for sale in the U.S.
2. Based on eight scans per day over 48 weeks.
3. Based on internal test results.



 March 06, 2026
March 06, 2026 









