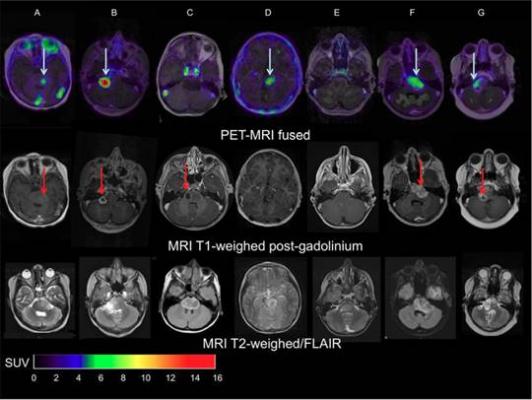
FIGURE: MRI AND PET-MRI FUSION IMAGES OF PATIENTS WITH DIPG. Top row: Zr-89-bevacizumab PET (144 hrs p.i.) fused with T1-Gd weighted MRI per patient; middle row: T1-Gd weighted MRI; lower row: T2-weighted/FLAIR MR-images. Five tumors show variable uptake of Zr-89-bevacizumab (white arrows), with both PET negative and positive areas within each tumor. Two primary tumors are completely PET negative (Fig. 1C and 1E), while the T2 weighted images show tumor infiltration in the whole pons of both patients. In the middle row, the red arrows represent the areas of contrast enhancement within the tumor. In four out of five primary tumors, the PET-positive area corresponds with the contrast-enhancing area on MRI of the tumors (Fig. 1A, 1B, 1F and 1G). In Fig. 1C, the tumor shows an MRI contrast-enhancing area, while there is no Zr-89-bevacizumab uptake. Fig. 1D shows a PET positive tumor, while no Gd-enhancement is observed on MRI. Credit: Sophie Veldhuijzen van Zanten and Marc Jansen, VU University Medical Center, Amsterdam, The Netherlands.
May 2, 2017 — In a first-ever molecular drug-imaging study in children, researchers in The Netherlands used whole-body positron emission tomography/computed tomography (PET/CT) scans to determine whether bevacizumab (Avastin) treatment of diffuse intrinsic pontine glioma (DIPG) in children is likely to be effective. The study is featured in the May 2017 issue of The Journal of Nuclear Medicine.
Brain cancers are difficult to treat, and it can be hard to predict whether a therapy will be effective. When the patient is a child, it is even more important to predict the potential effectiveness of a drug before beginning treatment.
“Children with DIPG have a very poor prognosis, with less than 10 percent of the patients surviving two years from diagnosis,” explained Guus A. van Dongen, Ph.D., of VU University, Medical Center, Amsterdam, The Netherlands. “These tumors are resistant to all kinds of therapies. Chemotherapy, as well as new targeted therapies, may not reach the tumor due to the location within the brainstem.”
For the study, researchers investigated whether bevacizumab can reach the tumor in children with DIPG by measuring the tumor uptake of zirconium-89 (Zr-89)-labeled bevacizumab with PET. In addition, they evaluated the safety of the procedure and determined the optimal timing of imaging.
Two weeks after completing radiotherapy, seven patients (age range: 6-17) were given whole-body PET/CT scans performed at 1, 72 and 144 hours post-injection. The optimal moment of scanning was found to be 144 hours post-injection. The patients also underwent contrast (gadolinium)-enhanced magnetic resonance imaging (MRI).
“The results showed that indeed there is considerable heterogeneity in uptake of Zr-89-labeled bevacizumab among patients and within tumors,” Van Dongen pointed out. “This non-invasive in vivo quantification of drug distribution and tumor uptake may help to predict therapeutic potential, as well as toxicity, and could help develop strategies for improving drug delivery to tumors.”
Van Dongen added, “Children with brain tumors and other solid cancers are particularly likely to benefit from molecular drug imaging, as drugs without therapeutic effect — based on a lack of drug-uptake in the tumor — may cause life-long side effects. Molecular drug imaging will open avenues for administering the right drug to the right patient at the most appropriate stage of the disease.”
For more information: www.jnm.snmjournals.org


 February 27, 2026
February 27, 2026 









