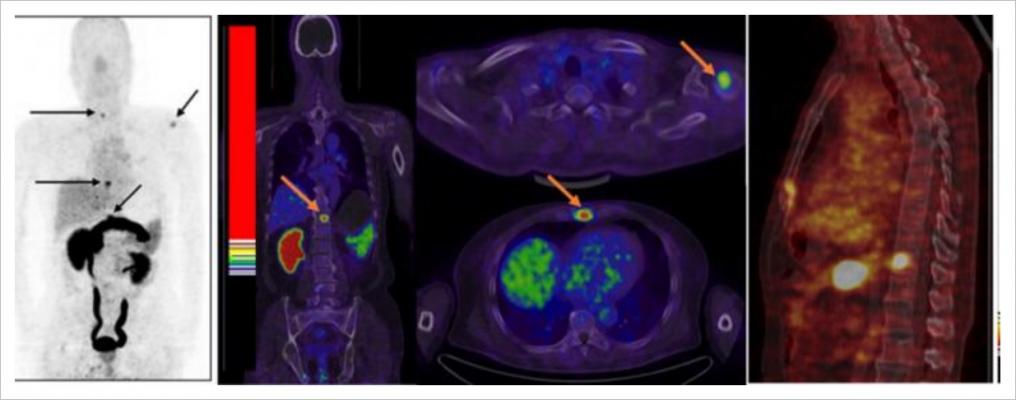
June 13, 2018 — A recently presented investigational clinical trial evaluated the impact of 18F fluciclovine positron emission tomography/computed tomography (PET/CT) imaging on patient management of biochemically recurrent prostate cancer after initial prostate cancer treatment and negative or equivocal findings on standard-of-care imaging. The LOCATE trial is a prospective, multi-center, open-label study (NCT02680041) conducted at 15 sites in the United States. Its primary endpoint measured the percentage of men with suspected biochemical recurrence of prostate cancer following initial prior therapy whose treatment plan was changed following an 18F fluciclovine PET/CT scan.
Axumin (fluciclovine F 18 injection) is a U.S. Food and Drug Administration (FDA)-approved molecular imaging agent for use in PET imaging in men with suspected prostate cancer recurrence; suspicion of prostate cancer recurrence is based on elevated blood levels of prostate specific antigen (PSA) following prior treatment.
The LOCATE trial recorded a patient’s intended treatment plan prior to 18F fluciclovine PET/CT and then recorded how it was altered after patients and their physicians had reviewed the results of the scan. Results of the trial indicated that 59 percent (126/213) of patients had their clinical management changed when results of the 18F fluciclovine PET/CT imaging were added to the diagnostic work-up. Of those changes, 78 percent (98/126) were classified as “major” (i.e., a change in treatment modality).
The topline results were presented in a Moderated Poster Session at the American Urological Association (AUA) 2018 annual meeting, May 18-21 in San Francisco. Results were presented by Gerald L. Andriole, M.D., Robert K. Royce Distinguished Professor and chief of urologic surgery at Washington University School of Medicine, St. Louis, on behalf of the LOCATE study group.
The LOCATE trial prospectively enrolled men with suspected biochemical recurrence of prostate cancer based on rising PSA levels following previous curative-intent treatment, and with negative or equivocal findings on standard-of-care imaging. The primary endpoint was to measure the percentage of patients with suspected biochemical recurrence of prostate cancer after therapy for primary disease whose planned treatment was altered following imaging with 18F fluciclovine PET/CT. Of the 128 patients whose original treatment plan was salvage radiation therapy (with or without androgen deprivation therapy [ADT]), 51 percent (65/128) had their treatment changed after 18F fluciclovine PET/CT. Of the 60 patients originally planned to be treated with ADT, 45/60 (75 percent) had their treatment changed to a non-systemic salvage treatment after 18F fluciclovine PET/CT; thirty of these were changed to a non-systemic salvage treatment and 11 to watchful waiting. The safety profile of 18F fluciclovine in the LOCATE trial is consistent with that described in the approved U.S. Prescribing Information.
“The results showed that management plans were revised for the majority of patients, and that 78 percent of such revisions involved a change in treatment modality. While investigation of the long-term clinical outcomes of these changes in management is warranted, these results indicate that decisions based on 18F fluciclovine PET/CT findings may facilitate appropriate management in men with suspected biochemical recurrence of prostate cancer,” said Andriole.
“Up to 30 percent of patients with prostate cancer will develop local or distant recurrences within 10 years of radical prostatectomy or radiation therapy, and determining the extent and location of recurrent disease optimizes the selection of appropriate treatment,” said Lale Kostakoglu, M.D., MPH, professor of radiology and chief of nuclear medicine, Icahn School of Medicine at Mount Sinai, New York, and member of one of the LOCATE study group’s leading enrollment sites. “The LOCATE study demonstrated that imaging with 18F fluciclovine PET/CT revealed one or more sites of disease recurrence in men with biochemically recurrent prostate cancer who were scanned, and based on the pattern of recurrence, resulted in a change in management after the scan in 59 percent of the patients.”
For more information: www.blueearthdiagnostics.com


 February 13, 2026
February 13, 2026 









