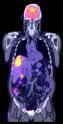
September 15, 2009 - Positron Corp. announced that the Ministry of Health and Long Term Care of Ontario, Canada is making positron emission tomography (PET) scanning a publicly insured health service available to cancer and cardiac patients under conditions where PET scans have been proven to be clinically effective. The Ministry of Health and Long Term Care (MOHLTC) has adopted an evidence-based approach to the introduction of PET imaging in the province for certain cancer and cardiac indications.
“Ontario, the largest province in Canada, introducing PET into their health system represents a significant opportunity for Positron to offer a cost effective general purpose scanner into a large socialized network,” said Joe Oliverio, chief technical officer of Positron. “We believe that the market for Positron’s Attrius PET scanner is potentially hundreds of units in a relatively short period of time. Health Canada’s approval of PET is further evidence that PET is the future of nuclear medicine. Positron plans to focus on marketing and selling its PET product throughout all Canadian provinces and estimates the entire market potential to be approximately 1,000 scanners.”
For more information: www.positron.com


 February 13, 2026
February 13, 2026 









