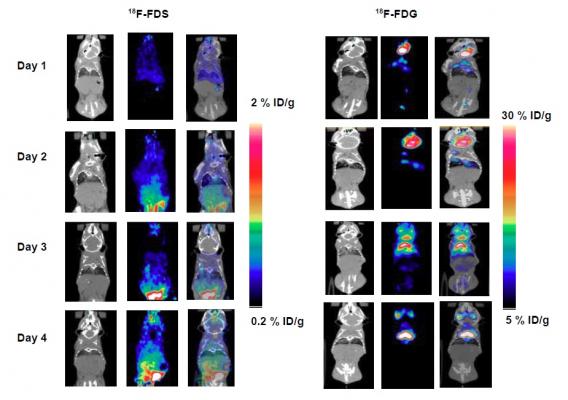
Representative PET/CT images of 18F-FDS and 18F-FDG in inflamed mice. Mice were inoculated with dead K. pneumoniae (10^8 CFU/mL). Imaging was performed for days 1, 2, 3 and 4 using 18F-FDG and 18F-FDS. CT images showed clear inflammation on day 2 and day 3 with corresponding high 18F-FDG uptake on PET. No significant uptake of 18F-FDS was detected for any of those 4 days. Credit: J Li et al., University of Louisville School of Medicine, Louisville, Ky.
January 22, 2018 — Researchers at the University of Louisville, Kentucky, have demonstrated that a new radiotracer, 2-18F-fluorodeoxysorbitol (18F-FDS), can identify and track bacterial infection in lungs better than current imaging methods and is able to differentiate bacterial infection from inflammation. The study is the featured basic science article in the January issue of The Journal of Nuclear Medicine.
“Currently, bacterial infections can be diagnosed only after they have become systemic or have caused significant anatomical tissue damage, a stage at which they are challenging to treat owing to the high bacterial burden,” explained Chin K. Ng, Ph.D., at the University of Louisville School of Medicine.
He pointed out, “18F-FDG PET, a widely commercially available imaging agent, is capable of imaging infection, but it cannot distinguish infections from other pathologies such as cancer and inflammation. Therefore, there is a great need to develop imaging agents with high specificity and sensitivity. There are still no specific imaging agents that can differentiate bacterial infection from sterile inflammation at an early stage.”
For this study, mice were inoculated with either live Klebsiella pneumoniae bacteria to induce lung infection, or the dead form of the bacteria to induce inflammation. Half of the mice with the live bacteria were imaged with positron emission tomography (PET)/computed tomography (CT) using either 18F-FDS or 18F-FDG on days 0, 1, 2 and 3 to monitor disease progression post-infection. The other half were screened by bioluminescent imaging, and mice with visible infection were selected for follow-up PET/CT scans with 18F-FDS. For the inflammation group, half the mice were imaged with PET/CT using 18F-FDS and half using 18F-FDG from day 1 to day 4 post-inoculation.
While both 18F-FDS and 18F-FDG effectively tracked the degree of bacterial infection measured by bioluminescent optical imaging, only 18F-FDS was able to differentiate lung infection from lung inflammation.
Ng noted, “Bacterial infection represents a threat to human health, including hospital-acquired, implant-related, and multidrug-resistant infections. 18F-FDS whole-body PET/CT imaging in mice has shown to be a unique imaging technique that could differentiate infection from inflammation. This same technique could potentially be used in patients to identify infection sites and determine the bacterial infection class, so that patients could avoid taking antibiotics that are known to have no effect against specific bacteria.”
He added, “The interpretation of CT appearances of lung disorders can be complex if a differential diagnosis needs to distinguish between inflammation and infection. Thus 18F-FDS PET/CT could be initially used as a follow up after an inconclusive CT diagnosis for suspected bacterial lung infection. As proven clinical data accumulate over time, 18F-FDS PET/CT could become a new clinical standard for confirming bacterial infection in the lungs or other sites.”
Looking ahead to making 18F-FDS clinically available, Ng stated, “Since 18F-FDS can be made from 18F-FDG with one extra, simple conversion step, and sorbitol has already been approved for use in humans by the U.S. Food and Drug Administration, the approval pathway for 18F-FDS should be straightforward. 18F-FDS would be inexpensive and readily available once approved.”
He also observed, “This and other new PET imaging agents demonstrate that molecular imaging and nuclear medicine can offer unique technologies for patient care and will continue to play a key influential role in healthcare.”
For more information: www.jnm.snmjournals.org


 February 13, 2026
February 13, 2026 









