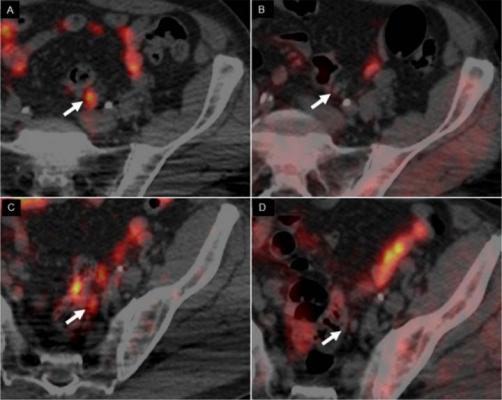
In scans of a 62-yr-old man with Gleason 4+3 PCa treated with radical prostatectomy, with rising PSA level (1.32) and PSA doubling time of 3.7 months, 64CuCl2-PET/CT images revealed 2 positive small left iliac lymph nodes (A,C), whereas 18F-Choline PET/CT (B,D) was negative (arrows). Image courtesy of A Piccardo et al., Galliera Hospital, Genoa, Italy.
An Italian study featured in the March issue of The Journal of Nuclear Medicine demonstrates that a novel nuclear medicine imaging agent targeting copper accumulation in tumors can detect prostate cancer recurrence early in patients with biochemical relapse (rising prostate-specific antigen [PSA] level).
Copper tends to be more concentrated in tumors, making it a good imaging biomarker. For this study of 50 patients, researchers conducted PET/CT scans comparing the new imaging agent, copper-64 chloride (64CuCl2), with fluorine-18-choline (18F-Choline). Multiparametric magnetic resonance imaging (mpMRI) was also conducted. In addition to calculating the detection rate of each imaging modality, the biodistribution, kinetics of the lesions and radiation dosimetry of 64CuCl2 were evaluated.
"This is the first time this novel agent has been compared with 18F-Choline-PET/CT in a considerable number of prostate cancer patients with biochemical relapse," explained Arnoldo Piccardo, of E.O. Ospedali Galliera in Genoa, Italy. He points out, "Early detection of prostate cancer relapse may improve the clinical management of patients, for example implementing early salvage radiotherapy."
The effective dose of 64CuCl2 was determined to be 5.7 mSv, similar to those of other established PET tracers (although higher than for 18F-Choline, which is 4 mSv). Unlike 18F-Choline, 64CuCl2 is neither accumulated in, nor excreted from, the urinary tract (main uptake is in the liver); this allows for thorough pelvic assessment, increasing the possibility of identifying small lesions close to the bladder. No adverse reactions were observed after the injection of 64CuCl2, and results show that 64CuCl2-PET/CT has a higher detection rate than 18F-Choline-PET/CT in patients with low levels of PSA (<1 ng/ml).
"This study determined that the biodistribution of 64CuCl2 is more suitable than that of 18F-Choline for exploring the pelvis and prostatic bed," said Piccardo. "In patients with biochemical relapse and a low PSA level, 64CuCl2-PET/CT shows a significantly higher detection rate than 18F-Choline-PET/CT." He reports, "Larger trials with this PET tracer are expected to further define its capabilities and role in the management of prostate cancer."
For mire information: http://jnm.snmjournals.org


 January 28, 2026
January 28, 2026 









