SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework.
Ensight is an extensible data management framework powered by artificial intelligence (AI) that allows easy implementation and orchestration of Enlitic application modules. Ensight facilitates the integration, deployment and management of AI-powered data management solutions.
Ensight 2.0 integrates ENDEXTM (data standardization) and ENCOGTM (data anonymization) into a single data management framework.
Using natural language processing and computer vision, Ensight helps improve radiologist reporting workflows, enhances data quality and creates new revenue opportunities for our customers.
 Ensight reviews information from DICOM pixel and metadata to identify key attributes of radiological examinations for standardization, and to detect and redact Protected Health Information found in the images. It contains basic functionality such as user access and security, dashboards for analytics and allows for integration with other devices such as third-party PACS software.
Ensight reviews information from DICOM pixel and metadata to identify key attributes of radiological examinations for standardization, and to detect and redact Protected Health Information found in the images. It contains basic functionality such as user access and security, dashboards for analytics and allows for integration with other devices such as third-party PACS software.
The output of this functionality is used for various healthcare workflows and enables healthcare providers to realize value from an increasing number of use cases that improves the data quality, such as identifying laterality conflicts or contrast billing discrepancies.
Key Features
This latest version of Ensight expands the suite of available use cases and provides additional capabilities.
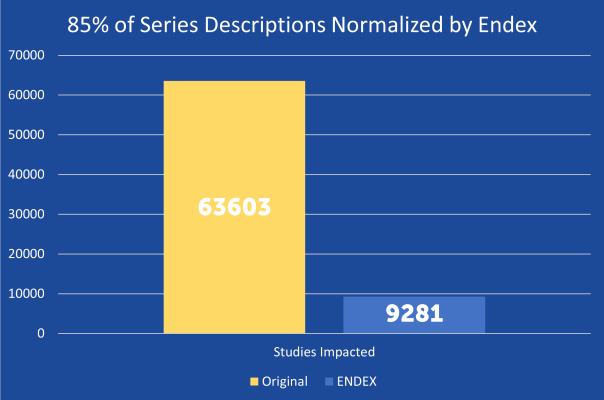
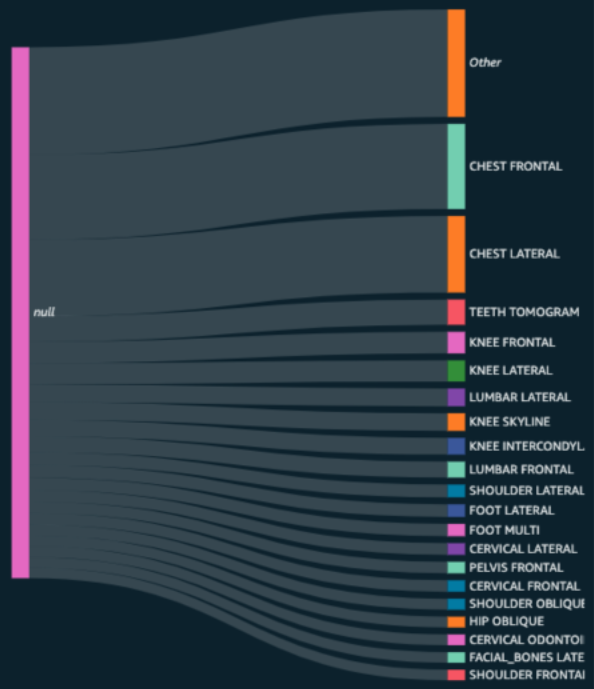
Among the key features Ensight 2.0 offers is improved dashboards and metrics to see how the system is performing and better evaluate the quality of the data.The AI models have been tuned to enable use of ontologies such as RSNA’s RadLex standard. The standardization of study/series descriptions can match RadLex descriptions.
Outputs are configurable to maintain the study and series characterization or can be enhanced with DICOM elements and/or the original elements. ENDEX can add any DICOM field to the study/series descriptions and it can maintain the original elements that users expect.
Integration with PACS is now enabled with HL7 messaging and DICOM SR. Relevant dictation templates can be launched by the HL7 message while PACS that require DICOM SR to accept the standardized study/series description can now do so.
Data Quality reports also will indicate the correctness, completeness and consistency of your data and identify where standardization can improve your data quality.
Setting the Stage
The newest version of Enlitic’s data standardization software framework was originally planned for the second half of 2025. According to Enlitic CEO Michael Sistenich, “The benefit of this [earlier] release will enable pending deployments and bring real value to our customers, while also setting the stage for us to deliver on our future roadmap.”
Click here to learn more about Ensight 2.0.
If you would like to schedule a demo to see how Enlitic can help enhance the quality of your medical imaging data using artificial intelligence, please click here.


 March 06, 2026
March 06, 2026 









